Dual role of the cysteine-string domain in membrane binding and palmitoylation-dependent sorting of the molecular chaperone cysteine-string protein
- PMID: 16943324
- PMCID: PMC1635403
- DOI: 10.1091/mbc.e06-03-0183
Dual role of the cysteine-string domain in membrane binding and palmitoylation-dependent sorting of the molecular chaperone cysteine-string protein
Abstract
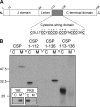
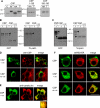
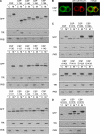
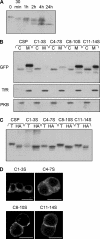
S-palmitoylation occurs on intracellular membranes and, therefore, membrane anchoring of proteins must precede palmitate transfer. However, a number of palmitoylated proteins lack any obvious membrane targeting motifs and it is unclear how this class of proteins become membrane associated before palmitoylation. Cysteine-string protein (CSP), which is extensively palmitoylated on a "string" of 14 cysteine residues, is an example of such a protein. In this study, we have investigated the mechanisms that govern initial membrane targeting, palmitoylation, and membrane trafficking of CSP. We identified a hydrophobic 31 amino acid domain, which includes the cysteine-string, as a membrane-targeting motif that associates predominantly with endoplasmic reticulum (ER) membranes. Cysteine residues in this domain are not merely sites for the addition of palmitate groups, but play an essential role in membrane recognition before palmitoylation. Membrane association of the cysteine-string domain is not sufficient to trigger palmitoylation, which requires additional downstream residues that may regulate the membrane orientation of the cysteine-string domain. CSP palmitoylation-deficient mutants remain "trapped" in the ER, suggesting that palmitoylation may regulate ER exit and correct intracellular sorting of CSP. These results reveal a dual function of the cysteine-string domain: initial membrane binding and palmitoylation-dependent sorting.
Figures


Similar articles
-
Palmitoylation and membrane interactions of the neuroprotective chaperone cysteine-string protein.J Biol Chem. 2008 Sep 5;283(36):25014-26. doi: 10.1074/jbc.M802140200. Epub 2008 Jul 2. J Biol Chem. 2008. PMID: 18596047 Free PMC article.
-
Cysteine-string protein isoform beta (Cspbeta) is targeted to the trans-Golgi network as a non-palmitoylated CSP in clonal beta-cells.Biochim Biophys Acta. 2007 Feb;1773(2):109-19. doi: 10.1016/j.bbamcr.2006.08.054. Epub 2006 Sep 14. Biochim Biophys Acta. 2007. PMID: 17034881
-
The cysteine-string domain of the secretory vesicle cysteine-string protein is required for membrane targeting.Biochem J. 1998 Oct 15;335 ( Pt 2)(Pt 2):205-9. doi: 10.1042/bj3350205. Biochem J. 1998. PMID: 9761715 Free PMC article.
-
Cysteine string protein (CSP) and its role in preventing neurodegeneration.Semin Cell Dev Biol. 2015 Apr;40:153-9. doi: 10.1016/j.semcdb.2015.03.008. Epub 2015 Mar 21. Semin Cell Dev Biol. 2015. PMID: 25800794 Free PMC article. Review.
-
Tying everything together: the multiple roles of cysteine string protein (CSP) in regulated exocytosis.Traffic. 2003 Oct;4(10):653-9. doi: 10.1034/j.1600-0854.2003.00127.x. Traffic. 2003. PMID: 12956868 Review.
Cited by
-
Proximity labelling reveals effects of disease-causing mutation on the DNAJC5/cysteine string protein α interactome.Biochem J. 2024 Jan 9;481(3):141-60. doi: 10.1042/BCJ20230319. Online ahead of print. Biochem J. 2024. PMID: 38193346 Free PMC article.
-
Palmitoylation-induced aggregation of cysteine-string protein mutants that cause neuronal ceroid lipofuscinosis.J Biol Chem. 2012 Oct 26;287(44):37330-9. doi: 10.1074/jbc.M112.389098. Epub 2012 Aug 19. J Biol Chem. 2012. PMID: 22902780 Free PMC article.
-
The roles of HSP40/DNAJ protein family in neurodegenerative diseases.Zhejiang Da Xue Xue Bao Yi Xue Ban. 2022 Nov 25;51(5):640-646. doi: 10.3724/zdxbyxb-2021-0406. Zhejiang Da Xue Xue Bao Yi Xue Ban. 2022. PMID: 36581576 Free PMC article. Review. English.
-
The large conductance, calcium-activated K+ (BK) channel is regulated by cysteine string protein.Sci Rep. 2013;3:2447. doi: 10.1038/srep02447. Sci Rep. 2013. PMID: 23945775 Free PMC article.
-
Identification of a Novel Sequence Motif Recognized by the Ankyrin Repeat Domain of zDHHC17/13 S-Acyltransferases.J Biol Chem. 2015 Sep 4;290(36):21939-50. doi: 10.1074/jbc.M115.657668. Epub 2015 Jul 21. J Biol Chem. 2015. PMID: 26198635 Free PMC article.
References
-
- Boal F., Zhang H., Tessier C., Scotti P., Lang J. The variable C-terminus of cysteine string proteins modulates exocytosis and protein-protein interactions. Biochemistry. 2004;43:16212–16223. - PubMed
-
- Braun J. E., Scheller R. H. Cysteine string protein, a DnaJ family member, is present on diverse secretory vesicles. Neuropharmacology. 1995;34:1361–1369. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

