Central efferent pathways mediating skin cooling-evoked sympathetic thermogenesis in brown adipose tissue
- PMID: 16931649
- PMCID: PMC2441894
- DOI: 10.1152/ajpregu.00427.2006
Central efferent pathways mediating skin cooling-evoked sympathetic thermogenesis in brown adipose tissue
Abstract
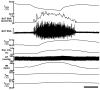
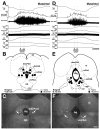
Control of thermoregulatory effectors by the autonomic nervous system is a critical component of rapid cold-defense responses, which are triggered by thermal information from the skin. However, the central autonomic mechanism driving thermoregulatory effector responses to skin thermal signals remains to be determined. Here, we examined the involvement of several autonomic brain regions in sympathetic thermogenic responses in brown adipose tissue (BAT) to skin cooling in urethane-chloralose-anesthetized rats by monitoring thermogenic [BAT sympathetic nerve activity (SNA) and BAT temperature], metabolic (expired CO(2)), and cardiovascular (arterial pressure and heart rate) parameters. Acute skin cooling, which did not reduce either rectal (core) or brain temperature, evoked increases in BAT SNA, BAT temperature, expired CO(2), and heart rate. Skin cooling-evoked thermogenic, metabolic, and heart rate responses were inhibited by bilateral microinjections of bicuculline (GABA(A) receptor antagonist) into the preoptic area (POA), by bilateral microinjections of muscimol (GABA(A) receptor agonist) into the dorsomedial hypothalamic nucleus (DMH), or by microinjection of muscimol, glycine, 8-OH-DPAT (5-HT(1A) receptor agonist), or kynurenate (nonselective antagonist for ionotropic excitatory amino acid receptors) into the rostral raphe pallidus nucleus (rRPa) but not by bilateral muscimol injections into the lateral/dorsolateral part or ventrolateral part of the caudal periaqueductal gray. These results implicate the POA, DMH, and rRPa in the central efferent pathways for thermogenic, metabolic, and cardiac responses to skin cooling, and suggest that these pathways can be modulated by serotonergic inputs to the medullary raphe.
Figures
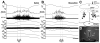
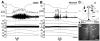

Comment in
-
The cold path to BAT.Am J Physiol Regul Integr Comp Physiol. 2007 Jan;292(1):R124-6. doi: 10.1152/ajpregu.00651.2006. Epub 2006 Sep 21. Am J Physiol Regul Integr Comp Physiol. 2007. PMID: 16990485 No abstract available.
Similar articles
-
Central efferent pathways for cold-defensive and febrile shivering.J Physiol. 2011 Jul 15;589(Pt 14):3641-58. doi: 10.1113/jphysiol.2011.210047. Epub 2011 May 24. J Physiol. 2011. PMID: 21610139 Free PMC article.
-
Excitatory amino acid receptor activation in the raphe pallidus area mediates prostaglandin-evoked thermogenesis.Neuroscience. 2003;122(1):5-15. doi: 10.1016/s0306-4522(03)00527-x. Neuroscience. 2003. PMID: 14596844
-
Medullary pathways mediating specific sympathetic responses to activation of dorsomedial hypothalamus.Neuroscience. 2004;126(1):229-40. doi: 10.1016/j.neuroscience.2004.03.013. Neuroscience. 2004. PMID: 15145088
-
The dorsomedial hypothalamus: a new player in thermoregulation.Am J Physiol Regul Integr Comp Physiol. 2007 Jan;292(1):R47-63. doi: 10.1152/ajpregu.00498.2006. Epub 2006 Sep 7. Am J Physiol Regul Integr Comp Physiol. 2007. PMID: 16959861 Review.
-
Efferent neural pathways for the control of brown adipose tissue thermogenesis and shivering.Handb Clin Neurol. 2018;156:281-303. doi: 10.1016/B978-0-444-63912-7.00017-5. Handb Clin Neurol. 2018. PMID: 30454595 Review.
Cited by
-
Induction of therapeutic hypothermia by pharmacological modulation of temperature-sensitive TRP channels: theoretical framework and practical considerations.Temperature (Austin). 2015 Apr 27;2(2):244-57. doi: 10.1080/23328940.2015.1024383. eCollection 2015 Apr-Jun. Temperature (Austin). 2015. PMID: 27227027 Free PMC article. Review.
-
Parabrachial opioidergic projections to preoptic hypothalamus mediate behavioral and physiological thermal defenses.Elife. 2021 Mar 5;10:e60779. doi: 10.7554/eLife.60779. Elife. 2021. PMID: 33667158 Free PMC article.
-
Integration of sensory information via central thermoregulatory leptin targets.Physiol Behav. 2013 Sep 10;121:49-55. doi: 10.1016/j.physbeh.2013.02.014. Epub 2013 Feb 28. Physiol Behav. 2013. PMID: 23458626 Free PMC article. Review.
-
Hypothalamic TRPV4 channels participate in the medial preoptic activation of warmth-defence responses in Wistar male rats.Pflugers Arch. 2019 Sep;471(9):1191-1203. doi: 10.1007/s00424-019-02303-1. Epub 2019 Aug 19. Pflugers Arch. 2019. PMID: 31428866
-
Mu and kappa opioid receptors of the periaqueductal gray stimulate and inhibit thermogenesis, respectively, during psychological stress in rats.Pflugers Arch. 2017 Sep;469(9):1151-1161. doi: 10.1007/s00424-017-1966-2. Epub 2017 Apr 4. Pflugers Arch. 2017. PMID: 28374069
References
-
- Bamshad M, Song CK, Bartness TJ. CNS origins of the sympathetic nervous system outflow to brown adipose tissue. Am J Physiol Regul Integr Comp Physiol. 1999;276:R1569–R1578. - PubMed
-
- Barnes NM, Sharp T. A review of central 5-HT receptors and their function. Neuropharmacol. 1999;38:1083–1152. - PubMed
-
- Berthoud HR, Patterson LM, Sutton GM, Morrison C, Zheng H. Orexin inputs to caudal raphe neurons involved in thermal, cardiovascular, and gastrointestinal regulation. Histochem Cell Biol. 2005;123:147–156. - PubMed
-
- Blier P, Seletti B, Gilbert F, Young SN, Benkelfat C. Serotonin 1A receptor activation and hypothermia in humans: lack of evidence for a presynaptic mediation. Neuropsychopharmacol. 2002;27:301–308. - PubMed
-
- Bonaz B, Taché Y. Induction of Fos immunoreactivity in the rat brain after cold-restraint induced gastric lesions and fecal excretion. Brain Res. 1994;652:56–64. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials

