Clathrin-dependent association of CVAK104 with endosomes and the trans-Golgi network
- PMID: 16914521
- PMCID: PMC1635376
- DOI: 10.1091/mbc.e06-05-0390
Clathrin-dependent association of CVAK104 with endosomes and the trans-Golgi network
Abstract
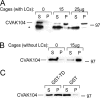
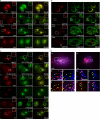
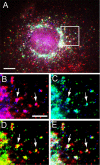
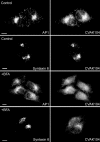
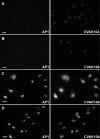
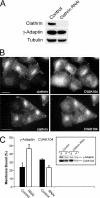
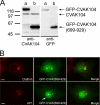
CVAK104 is a novel coated vesicle-associated protein with a serine/threonine kinase homology domain that was recently shown to phosphorylate the beta2-subunit of the adaptor protein (AP) complex AP2 in vitro. Here, we demonstrate that a C-terminal segment of CVAK104 interacts with the N-terminal domain of clathrin and with the alpha-appendage of AP2. CVAK104 localizes predominantly to the perinuclear region of HeLa and COS-7 cells, but it is also present on peripheral vesicular structures that are accessible to endocytosed transferrin. The distribution of CVAK104 overlaps extensively with that of AP1, AP3, the mannose 6-phosphate receptor, and clathrin but not at all with its putative phosphorylation target AP2. RNA interference-mediated clathrin knockdown reduced the membrane association of CVAK104. Recruitment of CVAK104 to perinuclear membranes of permeabilized cells is enhanced by guanosine 5'-O-(3-thio)triphosphate, and brefeldin A redistributes CVAK104 in cells. Both observations suggest a direct or indirect requirement for GTP-binding proteins in the membrane association of CVAK104. Live-cell imaging showed colocalization of green fluorescent protein-CVAK104 with endocytosed transferrin and with red fluorescent protein-clathrin on rapidly moving endosomes. Like AP1-depleted COS-7 cells, CVAK104-depleted cells missort the lysosomal hydrolase cathepsin D. Together, our data suggest a function for CVAK104 in clathrin-dependent pathways between the trans-Golgi network and the endosomal system.
Figures

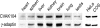

Similar articles
-
CVAK104 is a novel regulator of clathrin-mediated SNARE sorting.Traffic. 2007 Jul;8(7):893-903. doi: 10.1111/j.1600-0854.2007.00576.x. Traffic. 2007. PMID: 17587408 Free PMC article.
-
EpsinR: an AP1/clathrin interacting protein involved in vesicle trafficking.J Cell Biol. 2003 Jan 20;160(2):213-22. doi: 10.1083/jcb.200208023. Epub 2003 Jan 21. J Cell Biol. 2003. PMID: 12538641 Free PMC article.
-
Lowe syndrome protein OCRL1 interacts with clathrin and regulates protein trafficking between endosomes and the trans-Golgi network.Mol Biol Cell. 2005 Aug;16(8):3467-79. doi: 10.1091/mbc.e05-02-0120. Epub 2005 May 25. Mol Biol Cell. 2005. PMID: 15917292 Free PMC article.
-
Visualization of TGN-endosome trafficking in mammalian and Drosophila cells.Methods Enzymol. 2012;504:255-71. doi: 10.1016/B978-0-12-391857-4.00013-6. Methods Enzymol. 2012. PMID: 22264539 Review.
-
[Regulatory mechanisms of the clathrin adaptor molecules AP-1 and GGAs].Tanpakushitsu Kakusan Koso. 2008 Dec;53(16 Suppl):2046-52. Tanpakushitsu Kakusan Koso. 2008. PMID: 21038583 Review. Japanese. No abstract available.
Cited by
-
SCYL2 Protects CA3 Pyramidal Neurons from Excitotoxicity during Functional Maturation of the Mouse Hippocampus.J Neurosci. 2015 Jul 22;35(29):10510-22. doi: 10.1523/JNEUROSCI.2056-14.2015. J Neurosci. 2015. PMID: 26203146 Free PMC article.
-
Recessive mutations in SCYL2 cause a novel syndromic form of arthrogryposis in humans.Hum Genet. 2020 Apr;139(4):513-519. doi: 10.1007/s00439-020-02117-7. Epub 2020 Jan 20. Hum Genet. 2020. PMID: 31960134 Clinical Trial.
-
CVAK104 is a novel regulator of clathrin-mediated SNARE sorting.Traffic. 2007 Jul;8(7):893-903. doi: 10.1111/j.1600-0854.2007.00576.x. Traffic. 2007. PMID: 17587408 Free PMC article.
-
Characterization of DNA variants in the human kinome in breast cancer.Sci Rep. 2015 Sep 30;5:14736. doi: 10.1038/srep14736. Sci Rep. 2015. PMID: 26420498 Free PMC article.
-
RNAi screening reveals a large signaling network controlling the Golgi apparatus in human cells.Mol Syst Biol. 2012;8:629. doi: 10.1038/msb.2012.59. Mol Syst Biol. 2012. PMID: 23212246 Free PMC article.
References
-
- Bar-Zvi D., Branton D. Clathrin-coated vesicles contain two protein kinase activities. Phosphorylation of clathrin beta-light chain by casein kinase II. J. Biol. Chem. 1986;261:9614–9621. - PubMed
-
- Behnia R., Munro S. Organelle identity and the signposts for membrane traffic. Nature. 2005;438:597–604. - PubMed
-
- Benesch S., Polo S., Lai F. P., Anderson K. I., Stradal T. E., Wehland J., Rottner K. N-WASP deficiency impairs EGF internalization and actin assembly at clathrin-coated pits. J. Cell Sci. 2005;118:3103–3115. - PubMed
-
- Berger E. G., Aegerter E., Mandel T., Hauri H. P. Monoclonal antibodies to soluble, human milk galactosyltransferase (lactose synthase A protein) Carbohydr. Res. 1986;149:23–33. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials

