Integrin structures and conformational signaling
- PMID: 16904883
- PMCID: PMC1618925
- DOI: 10.1016/j.ceb.2006.08.005
Integrin structures and conformational signaling
Abstract
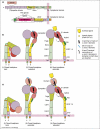
Integrins are cell adhesion molecules that play critical roles in development, wound healing, hemostasis, immunity and cancer. Advances in the past two years have shed light on the structural basis for integrin regulation and signaling, especially on how global conformational changes between bent and extended conformations relate to the inter-domain and intra-domain shape shifting that regulates affinity for ligand. The downward movements of the C-terminal helices of the alpha I and beta I domains and the swing-out of the hybrid domain play pivotal roles in integrin conformational signaling. Experiments have also shown that integrins transmit bidirectional signals across the plasma membrane by coupling extracellular conformational change with an unclasping and separation of the alpha and beta transmembrane and cytoplasmic domains.
Figures
Similar articles
-
Overview: structural biology of integrins.Methods Mol Biol. 2012;757:81-99. doi: 10.1007/978-1-61779-166-6_7. Methods Mol Biol. 2012. PMID: 21909908 Review.
-
Global conformational rearrangements in integrin extracellular domains in outside-in and inside-out signaling.Cell. 2002 Sep 6;110(5):599-11. doi: 10.1016/s0092-8674(02)00935-2. Cell. 2002. PMID: 12230977
-
Structure and mechanics of integrin-based cell adhesion.Curr Opin Cell Biol. 2007 Oct;19(5):495-507. doi: 10.1016/j.ceb.2007.08.002. Epub 2007 Oct 24. Curr Opin Cell Biol. 2007. PMID: 17928215 Free PMC article. Review.
-
Atypical structure and function of integrin αV β8.J Cell Physiol. 2021 Jul;236(7):4874-4887. doi: 10.1002/jcp.30242. Epub 2020 Dec 27. J Cell Physiol. 2021. PMID: 33368230 Review.
-
Determination of N- and C-terminal borders of the transmembrane domain of integrin subunits.J Biol Chem. 2004 May 14;279(20):21200-5. doi: 10.1074/jbc.M400771200. Epub 2004 Mar 10. J Biol Chem. 2004. PMID: 15016834
Cited by
-
Force regulated conformational change of integrin αVβ3.Matrix Biol. 2017 Jul;60-61:70-85. doi: 10.1016/j.matbio.2016.07.002. Epub 2016 Jul 14. Matrix Biol. 2017. PMID: 27423389 Free PMC article.
-
Mechanisms of assembly and remodelling of the extracellular matrix.Nat Rev Mol Cell Biol. 2024 Nov;25(11):865-885. doi: 10.1038/s41580-024-00767-3. Epub 2024 Sep 2. Nat Rev Mol Cell Biol. 2024. PMID: 39223427 Review.
-
beta2-integrins in demyelinating disease: not adhering to the paradigm.J Leukoc Biol. 2010 Mar;87(3):397-403. doi: 10.1189/jlb.1009654. Epub 2009 Dec 9. J Leukoc Biol. 2010. PMID: 20007244 Free PMC article. Review.
-
Three-dimensional model of human platelet integrin alphaIIb beta3 in solution obtained by small angle neutron scattering.J Biol Chem. 2010 Jan 8;285(2):1023-31. doi: 10.1074/jbc.M109.050039. Epub 2009 Nov 6. J Biol Chem. 2010. PMID: 19897481 Free PMC article.
-
Talin and Kindlin as Integrin-Activating Proteins: Focus on the Heart.Pediatr Cardiol. 2019 Oct;40(7):1401-1409. doi: 10.1007/s00246-019-02167-3. Epub 2019 Jul 31. Pediatr Cardiol. 2019. PMID: 31367953 Free PMC article. Review.
References
-
- Xiong JP, Stehle T, Zhang R, Joachimiak A, Frech M, Goodman SL, Arnaout MA. Crystal structure of the extracellular segment of integrin αVβ3 in complex with an Arg-Gly-Asp ligand. Science. 2002;296:151–155. - PubMed
-
- Takagi J, Petre BM, Walz T, Springer TA. Global conformational rearrangements in integrin extracellular domains in outside-in and inside-out signaling. Cell. 2002;110:599–611. - PubMed
-
- Kim M, Carman CV, Springer TA. Bidirectional transmembrane signaling by cytoplasmic domain separation in integrins. Science. 2003;301:1720–1725. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

