Mps1 phosphorylation of Dam1 couples kinetochores to microtubule plus ends at metaphase
- PMID: 16890524
- PMCID: PMC1762913
- DOI: 10.1016/j.cub.2006.06.063
Mps1 phosphorylation of Dam1 couples kinetochores to microtubule plus ends at metaphase
Abstract
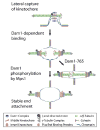
Background: Duplicated chromosomes are equally segregated to daughter cells by a bipolar mitotic spindle during cell division. By metaphase, sister chromatids are coupled to microtubule (MT) plus ends from opposite poles of the bipolar spindle via kinetochores. Here we describe a phosphorylation event that promotes the coupling of kinetochores to microtubule plus ends.
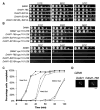
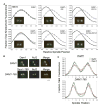
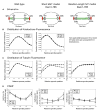
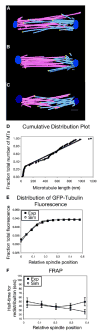
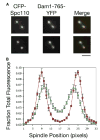
Results: Dam1 is a kinetochore component that directly binds to microtubules. We identified DAM1-765, a dominant allele of DAM1, in a genetic screen for mutations that increase stress on the spindle pole body (SPB) in Saccharomyces cerevisiae. DAM1-765 contains the single mutation S221F. We show that S221 is one of six Dam1 serines (S13, S49, S217, S218, S221, and S232) phosphorylated by Mps1 in vitro. In cells with single mutations S221F, S218A, or S221A, kinetochores in the metaphase spindle form tight clusters that are closer to the SPBs than in a wild-type cell. Five lines of experimental evidence, including localization of spindle components by fluorescence microscopy, measurement of microtubule dynamics by fluorescence redistribution after photobleaching, and reconstructions of three-dimensional structure by electron tomography, combined with computational modeling of microtubule behavior strongly indicate that, unlike wild-type kinetochores, Dam1-765 kinetochores do not colocalize with an equal number of plus ends. Despite the uncoupling of the kinetochores from the plus ends of MTs, the DAM1-765 cells are viable, complete the cell cycle with the same kinetics as wild-type cells, and biorient their chromosomes as efficiently as wild-type cells.
Conclusions: We conclude that phosphorylation of Dam1 residues S218 and S221 by Mps1 is required for efficient coupling of kinetochores to MT plus ends. We find that efficient plus-end coupling is not required for (1) maintenance of chromosome biorientation, (2) maintenance of tension between sister kinetochores, or (3) chromosome segregation.
Figures

Similar articles
-
Laterally attached kinetochores recruit the checkpoint protein Bub1, but satisfy the spindle checkpoint.Cell Cycle. 2010 Sep 1;9(17):3619-28. doi: 10.4161/cc.9.17.12907. Cell Cycle. 2010. PMID: 20928940 Free PMC article.
-
Mps1 promotes chromosome meiotic chromosome biorientation through Dam1.Mol Biol Cell. 2018 Feb 15;29(4):479-489. doi: 10.1091/mbc.E17-08-0503. Epub 2017 Dec 13. Mol Biol Cell. 2018. PMID: 29237818 Free PMC article.
-
Chromosome segregation: Mps1 and Dam1 battle to bind a shared interaction site at the kinetochore.Curr Biol. 2024 Jun 3;34(11):R530-R533. doi: 10.1016/j.cub.2024.04.070. Curr Biol. 2024. PMID: 38834024
-
Merotelic kinetochores in mammalian tissue cells.Philos Trans R Soc Lond B Biol Sci. 2005 Mar 29;360(1455):553-68. doi: 10.1098/rstb.2004.1610. Philos Trans R Soc Lond B Biol Sci. 2005. PMID: 15897180 Free PMC article. Review.
-
Killing two birds with one stone: how budding yeast Mps1 controls chromosome segregation and spindle assembly checkpoint through phosphorylation of a single kinetochore protein.Curr Genet. 2020 Dec;66(6):1037-1044. doi: 10.1007/s00294-020-01091-x. Epub 2020 Jul 6. Curr Genet. 2020. PMID: 32632756 Review.
Cited by
-
Leader of the SAC: molecular mechanisms of Mps1/TTK regulation in mitosis.Open Biol. 2018 Aug;8(8):180109. doi: 10.1098/rsob.180109. Open Biol. 2018. PMID: 30111590 Free PMC article. Review.
-
A Gradient in Metaphase Tension Leads to a Scaled Cellular Response in Mitosis.Dev Cell. 2019 Apr 8;49(1):63-76.e10. doi: 10.1016/j.devcel.2019.01.018. Epub 2019 Feb 21. Dev Cell. 2019. PMID: 30799228 Free PMC article.
-
Chromosome segregation in budding yeast: sister chromatid cohesion and related mechanisms.Genetics. 2014 Jan;196(1):31-63. doi: 10.1534/genetics.112.145144. Genetics. 2014. PMID: 24395824 Free PMC article. Review.
-
An interaction hub on Ndc80 complex facilitates dynamic recruitment of Mps1 to yeast kinetochores to promote accurate chromosome segregation.bioRxiv [Preprint]. 2023 Nov 7:2023.11.07.566082. doi: 10.1101/2023.11.07.566082. bioRxiv. 2023. Update in: Curr Biol. 2024 Jun 3;34(11):2294-2307.e4. doi: 10.1016/j.cub.2024.04.054. PMID: 37986816 Free PMC article. Updated. Preprint.
-
Dissecting the role of MPS1 in chromosome biorientation and the spindle checkpoint through the small molecule inhibitor reversine.J Cell Biol. 2010 Jul 12;190(1):73-87. doi: 10.1083/jcb.201001036. J Cell Biol. 2010. PMID: 20624901 Free PMC article.
References
-
- Merdes A, De Mey J. The mechanism of kinetochore-spindle attachment and polewards movement analyzed in PtK2 cells at the prophase-prometaphase transition. Eur J Cell Biol. 1990;53:313–325. - PubMed
-
- Tanaka K, Mukae N, Dewar H, van Breugel M, James EK, Prescott AR, Antony C, Tanaka TU. Molecular mechanisms of kinetochore capture by spindle microtubules. Nature. 2005;434:987–994. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

