Population dynamics of sporogony for Plasmodium vivax parasites from western Thailand developing within three species of colonized Anopheles mosquitoes
- PMID: 16887043
- PMCID: PMC1557861
- DOI: 10.1186/1475-2875-5-68
Population dynamics of sporogony for Plasmodium vivax parasites from western Thailand developing within three species of colonized Anopheles mosquitoes
Abstract
Background: The population dynamics of Plasmodium sporogony within mosquitoes consists of an early phase where parasite abundance decreases during the transition from gametocyte to oocyst, an intermediate phase where parasite abundance remains static as oocysts, and a later phase where parasite abundance increases during the release of progeny sporozoites from oocysts. Sporogonic development is complete when sporozoites invade the mosquito salivary glands. The dynamics and efficiency of this developmental sequence were determined in laboratory strains of Anopheles dirus, Anopheles minimus and Anopheles sawadwongporni mosquitoes for Plasmodium vivax parasites circulating naturally in western Thailand.
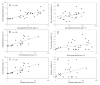
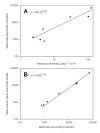
Methods: Mosquitoes were fed blood from 20 symptomatic Thai adults via membrane feeders. Absolute densities were estimated for macrogametocytes, round stages (= female gametes/zygotes), ookinetes, oocysts, haemolymph sporozoites and salivary gland sporozoites. From these census data, five aspects of population dynamics were analysed; 1) changes in life-stage prevalence during early sporogony, 2) kinetics of life-stage formation, 3) efficiency of life-stage transitions, 4) density relationships between successive life-stages, and 5) parasite aggregation patterns.
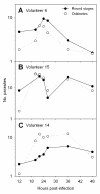
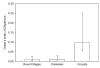
Results: There was no difference among the three mosquito species tested in total losses incurred by P. vivax populations during early sporogony. Averaged across all infections, parasite populations incurred a 68-fold loss in abundance, with losses of ca. 19-fold, 2-fold and 2-fold at the first (= gametogenesis/fertilization), second (= round stage transformation), and third (= ookinete migration) life-stage transitions, respectively. However, total losses varied widely among infections, ranging from 6-fold to over 2,000-fold loss. Losses during gametogenesis/fertilization accounted for most of this variability, indicating that gametocytes originating from some volunteers were more fertile than those from other volunteers. Although reasons for such variability were not determined, gametocyte fertility was not correlated with blood haematocrit, asexual parasitaemia, gametocyte density or gametocyte sex ratio. Round stages and ookinetes were present in mosquito midguts for up to 48 hours and development was asynchronous. Parasite losses during fertilization and round stage differentiation were more influenced by factors intrinsic to the parasite and/or factors in the blood, whereas ookinete losses were more strongly influenced by mosquito factors. Oocysts released sporozoites on days 12 to 14, but even by day 22 many oocysts were still present on the midgut. The per capita production was estimated to be approximately 500 sporozoites per oocyst and approximately 75% of the sporozoites released into the haemocoel successfully invaded the salivary glands.
Conclusion: The major developmental bottleneck in early sporogony occurred during the transition from macrogametocyte to round stage. Sporozoite invasion into the salivary glands was very efficient. Information on the natural population dynamics of sporogony within malaria-endemic areas may benefit intervention strategies that target early sporogony (e.g., transmission blocking vaccines, transgenic mosquitoes).
Figures


Similar articles
-
Population dynamics of Plasmodium falciparum sporogony in laboratory-infected Anopheles gambiae.J Parasitol. 1992 Aug;78(4):716-24. J Parasitol. 1992. PMID: 1635032
-
Susceptibility of Anopheles sinensis to Plasmodium vivax in malarial outbreak areas of central China.Parasit Vectors. 2013 Jun 14;6:176. doi: 10.1186/1756-3305-6-176. Parasit Vectors. 2013. PMID: 23768077 Free PMC article.
-
Rodent Plasmodium: population dynamics of early sporogony within Anopheles stephensi mosquitoes.J Parasitol. 2008 Oct;94(5):999-1008. doi: 10.1645/GE-1407.1. J Parasitol. 2008. PMID: 18576764
-
Malaria parasite development in mosquitoes.Annu Rev Entomol. 1998;43:519-43. doi: 10.1146/annurev.ento.43.1.519. Annu Rev Entomol. 1998. PMID: 9444756 Review.
-
Getting infectious: formation and maturation of Plasmodium sporozoites in the Anopheles vector.Cell Microbiol. 2006 Oct;8(10):1547-56. doi: 10.1111/j.1462-5822.2006.00778.x. Cell Microbiol. 2006. PMID: 16984410 Review.
Cited by
-
Late sporogonic stages of Plasmodium parasites are susceptible to the melanization response in Anopheles gambiae mosquitoes.Front Cell Infect Microbiol. 2024 Aug 1;14:1438019. doi: 10.3389/fcimb.2024.1438019. eCollection 2024. Front Cell Infect Microbiol. 2024. PMID: 39149419 Free PMC article.
-
Late sporogonic stages of Plasmodium parasites are susceptible to the melanization response in Anopheles gambiae mosquitoes.bioRxiv [Preprint]. 2024 May 31:2024.05.31.596773. doi: 10.1101/2024.05.31.596773. bioRxiv. 2024. Update in: Front Cell Infect Microbiol. 2024 Aug 01;14:1438019. doi: 10.3389/fcimb.2024.1438019. PMID: 38853990 Free PMC article. Updated. Preprint.
-
In-vitro susceptibility and ex-vivo evaluation of macrocyclic lactone endectocides sub-lethal concentrations against Plasmodium vivax oocyst development in Anopheles arabiensis.Malar J. 2024 Jan 18;23(1):26. doi: 10.1186/s12936-024-04845-x. Malar J. 2024. PMID: 38238768 Free PMC article.
-
A Pvs25 mRNA vaccine induces complete and durable transmission-blocking immunity to Plasmodium vivax.NPJ Vaccines. 2023 Dec 14;8(1):187. doi: 10.1038/s41541-023-00786-9. NPJ Vaccines. 2023. PMID: 38092803 Free PMC article.
-
Transmission-blocking activity of antimalarials for Plasmodium vivax malaria in Anopheles darlingi.PLoS Negl Trop Dis. 2023 Jun 16;17(6):e0011425. doi: 10.1371/journal.pntd.0011425. eCollection 2023 Jun. PLoS Negl Trop Dis. 2023. PMID: 37327209 Free PMC article.
References
-
- Sinden RE. Plasmodium differentiation in the mosquito. Parassitologia. 1999;41:139–148. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources

