Eclipse phase of herpes simplex virus type 1 infection: Efficient dynein-mediated capsid transport without the small capsid protein VP26
- PMID: 16873277
- PMCID: PMC1563788
- DOI: 10.1128/JVI.02528-05
Eclipse phase of herpes simplex virus type 1 infection: Efficient dynein-mediated capsid transport without the small capsid protein VP26
Abstract
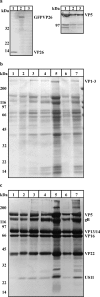
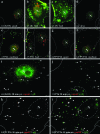
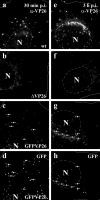
Cytoplasmic dynein,together with its cofactor dynactin, transports incoming herpes simplex virus type 1 (HSV-1) capsids along microtubules (MT) to the MT-organizing center (MTOC). From the MTOC, capsids move further to the nuclear pore, where the viral genome is released into the nucleoplasm. The small capsid protein VP26 can interact with the dynein light chains Tctex1 (DYNLT1) and rp3 (DYNLT3) and may recruit dynein to the capsid. Therefore, we analyzed nuclear targeting of incoming HSV1-DeltaVP26 capsids devoid of VP26 and of HSV1-GFPVP26 capsids expressing a GFPVP26 fusion instead of VP26. To compare the cell entry of different strains, we characterized the inocula with respect to infectivity, viral genome content, protein composition, and particle composition. Preparations with a low particle-to-PFU ratio showed efficient nuclear targeting and were considered to be of higher quality than those containing many defective particles, which were unable to induce plaque formation. When cells were infected with HSV-1 wild type, HSV1-DeltaVP26, or HSV1-GFPVP26, viral capsids were transported along MT to the nucleus. Moreover, when dynein function was inhibited by overexpression of the dynactin subunit dynamitin, fewer capsids of HSV-1 wild type, HSV1-DeltaVP26, and HSV1-GFPVP26 arrived at the nucleus. Thus, even in the absence of the potential viral dynein receptor VP26, HSV-1 used MT and dynein for efficient nuclear targeting. These data suggest that besides VP26, HSV-1 encodes other receptors for dynein or dynactin.
Figures

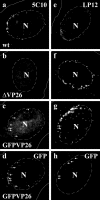

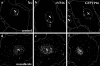
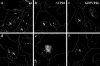
Similar articles
-
Function of dynein and dynactin in herpes simplex virus capsid transport.Mol Biol Cell. 2002 Aug;13(8):2795-809. doi: 10.1091/mbc.01-07-0348. Mol Biol Cell. 2002. PMID: 12181347 Free PMC article.
-
Herpes simplex virus type 1 capsid protein VP26 interacts with dynein light chains RP3 and Tctex1 and plays a role in retrograde cellular transport.J Biol Chem. 2004 Jul 2;279(27):28522-30. doi: 10.1074/jbc.M311671200. Epub 2004 Apr 26. J Biol Chem. 2004. PMID: 15117959
-
Plus- and minus-end directed microtubule motors bind simultaneously to herpes simplex virus capsids using different inner tegument structures.PLoS Pathog. 2010 Jul 8;6(7):e1000991. doi: 10.1371/journal.ppat.1000991. PLoS Pathog. 2010. PMID: 20628567 Free PMC article.
-
Transport and egress of herpes simplex virus in neurons.Rev Med Virol. 2008 Jan-Feb;18(1):35-51. doi: 10.1002/rmv.560. Rev Med Virol. 2008. PMID: 17992661 Review.
-
The association of viral proteins with host cell dynein components during virus infection.FEBS J. 2011 Sep;278(17):2997-3011. doi: 10.1111/j.1742-4658.2011.08252.x. Epub 2011 Aug 8. FEBS J. 2011. PMID: 21777384 Free PMC article. Review.
Cited by
-
The herpesvirus VP1/2 protein is an effector of dynein-mediated capsid transport and neuroinvasion.Cell Host Microbe. 2013 Feb 13;13(2):193-203. doi: 10.1016/j.chom.2013.01.009. Cell Host Microbe. 2013. PMID: 23414759 Free PMC article.
-
A hitchhiker's guide to the nervous system: the complex journey of viruses and toxins.Nat Rev Microbiol. 2010 Sep;8(9):645-55. doi: 10.1038/nrmicro2395. Nat Rev Microbiol. 2010. PMID: 20706281 Review.
-
The major determinant for addition of tegument protein pUL48 (VP16) to capsids in herpes simplex virus type 1 is the presence of the major tegument protein pUL36 (VP1/2).J Virol. 2010 Feb;84(3):1397-405. doi: 10.1128/JVI.01721-09. Epub 2009 Nov 18. J Virol. 2010. PMID: 19923173 Free PMC article.
-
Uncoupling uncoating of herpes simplex virus genomes from their nuclear import and gene expression.J Virol. 2011 May;85(9):4271-83. doi: 10.1128/JVI.02067-10. Epub 2011 Feb 23. J Virol. 2011. PMID: 21345968 Free PMC article.
-
HSV-1 Cgal+ infection promotes quaking RNA binding protein production and induces nuclear-cytoplasmic shuttling of quaking I-5 isoform in human hepatoma cells.Mol Cell Proteomics. 2011 Jun;10(6):M111.009126. doi: 10.1074/mcp.M111.009126. Epub 2011 Apr 5. Mol Cell Proteomics. 2011. PMID: 21467216 Free PMC article.
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

