Chs5/6 complex: a multiprotein complex that interacts with and conveys chitin synthase III from the trans-Golgi network to the cell surface
- PMID: 16855022
- PMCID: PMC1635352
- DOI: 10.1091/mbc.e06-03-0210
Chs5/6 complex: a multiprotein complex that interacts with and conveys chitin synthase III from the trans-Golgi network to the cell surface
Abstract
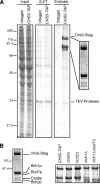
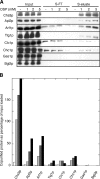
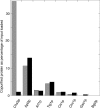
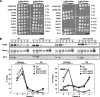
In Saccharomyces cerevisiae, the polysaccharide chitin is deposited at the mother bud junction by an integral membrane enzyme, chitin synthase 3 (Chs3p). The traffic of Chs3p to the cell surface from the trans-Golgi network (TGN) depends on two proteins, Chs5p and Chs6p, which sort selected cargo proteins into secretory vesicles. We have found that Chs5p forms a large higher-order complex of around 1 MDa with Chs6p and three Chs6 paralogs: Bch1p, Bud7p, and Bch2p. The Chs5/6 complex transiently interacts with its cargo, Chs3p, and the presence of Chs3p in the complex is dependent on every subunit. Chs5p and Chs6p have unique and crucial roles in Chs3p transport because either a chs5delta or chs6delta mutant drastically reduces the level of Chs3p bound to the remaining subunits of the complex. Bch1p and Bud7p appear to have a redundant function in Chs3p transport because deletion of both is necessary to displace Chs3p from the complex. The role of Bch2p in Chs3p binding is the least important. Chs5p is essential for structural integrity of the Chs5/6 complex and may act as a scaffold through which the other subunits assemble. Our results suggest a model of protein sorting at the TGN that involves a peripheral, possibly coat, complex that includes multiple related copies of a specificity determining subunit.
Figures


Similar articles
-
The complex interactions of Chs5p, the ChAPs, and the cargo Chs3p.Mol Biol Cell. 2012 Nov;23(22):4402-15. doi: 10.1091/mbc.E11-12-1015. Epub 2012 Sep 26. Mol Biol Cell. 2012. PMID: 23015758 Free PMC article.
-
Exomer: A coat complex for transport of select membrane proteins from the trans-Golgi network to the plasma membrane in yeast.J Cell Biol. 2006 Sep 25;174(7):973-83. doi: 10.1083/jcb.200605106. J Cell Biol. 2006. PMID: 17000877 Free PMC article.
-
Arf1p, Chs5p and the ChAPs are required for export of specialized cargo from the Golgi.EMBO J. 2006 Mar 8;25(5):943-54. doi: 10.1038/sj.emboj.7601007. Epub 2006 Feb 23. EMBO J. 2006. PMID: 16498409 Free PMC article.
-
Lipid-dependent protein sorting at the trans-Golgi network.Biochim Biophys Acta. 2012 Aug;1821(8):1059-67. doi: 10.1016/j.bbalip.2011.12.008. Epub 2011 Dec 31. Biochim Biophys Acta. 2012. PMID: 22230596 Review.
-
Domains of the TGN: coats, tethers and G proteins.Traffic. 2004 May;5(5):315-26. doi: 10.1111/j.1398-9219.2004.00182.x. Traffic. 2004. PMID: 15086781 Review.
Cited by
-
Architecture and biosynthesis of the Saccharomyces cerevisiae cell wall.Genetics. 2012 Nov;192(3):775-818. doi: 10.1534/genetics.112.144485. Genetics. 2012. PMID: 23135325 Free PMC article. Review.
-
Identification of New Antifungal Agents Targeting Chitin Synthesis by a Chemical-Genetic Method.Molecules. 2019 Aug 29;24(17):3155. doi: 10.3390/molecules24173155. Molecules. 2019. PMID: 31470665 Free PMC article.
-
The Arf-GAP Age2 localizes to the late-Golgi via a conserved amphipathic helix.Mol Biol Cell. 2023 Nov 1;34(12):ar119. doi: 10.1091/mbc.E23-07-0283. Epub 2023 Sep 6. Mol Biol Cell. 2023. PMID: 37672345 Free PMC article.
-
Induction of membrane curvature by proteins involved in Golgi trafficking.Adv Biol Regul. 2020 Jan;75:100661. doi: 10.1016/j.jbior.2019.100661. Epub 2019 Oct 16. Adv Biol Regul. 2020. PMID: 31668661 Free PMC article. Review.
-
The exomer coat complex transports Fus1p to the plasma membrane via a novel plasma membrane sorting signal in yeast.Mol Biol Cell. 2009 Dec;20(23):4985-96. doi: 10.1091/mbc.e09-04-0324. Epub 2009 Oct 7. Mol Biol Cell. 2009. PMID: 19812245 Free PMC article.
References
-
- Ausubel F. M., Brent R., Kingston R. E., Moore D. D., Seidman J. G., Smith J. A., Struhl K. Current Protocols in Molecular Biology. New York: John Wiley & Sons; 1987–1995.
-
- Bonifacino J. S., Lippincott-Schwartz J. Coat proteins: shaping membrane transport. Nat. Rev. Mol. Cell Biol. 2003;4:409–414. - PubMed
-
- Bulawa C. E. Genetics and molecular biology of chitin synthesis in fungi. Annu. Rev. Microbiol. 1993;47:505–534. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Miscellaneous

