Calcium-dependent enhancement of transcription of p300 by human T-lymphotropic type 1 p12I
- PMID: 16843515
- PMCID: PMC3044894
- DOI: 10.1016/j.virol.2006.06.005
Calcium-dependent enhancement of transcription of p300 by human T-lymphotropic type 1 p12I
Abstract
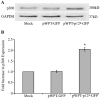
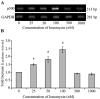
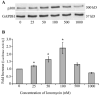
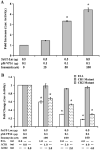
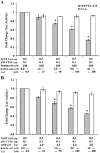
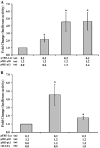
Human T-lymphotropic virus type 1 (HTLV-1) p12I localizes to the endoplasmic reticulum and Golgi causing sustained release of calcium, T cell activation, and enhanced expression of several calcium-regulated genes. In recent microarray studies, p300 mRNA was increased in T cells expressing p12I. The co-activator p300 is a key regulator of cellular and viral transcription; however, factors that influence its transcriptional regulation are less well studied. We hypothesized that the transcription of p300 is calcium dependent and that sustained low magnitude increases in intracellular calcium may enhance the transcription of p300. Herein, we report enhanced expression of p300 in T cells by p12I in a calcium-dependent, but calcineurin-independent manner. Sustained low magnitude calcium release induced by ionomycin in T cells was sufficient to increased mRNA and protein levels of p300 resulting in enhanced transcription from a p300-dependent promoter. Promoter analysis of the p300 gene was used to predict calcium-responsive transcription factor binding sites. Using mutant forms of p12I, we demonstrate that ER localization of the viral protein is required to increase p300. In addition, p12I reversed the repression of HTLV-1 LTR-driven transcription by HTLV-1 p30II, a p300-binding protein. HTLV-1 p12I-mediated enhancement of p300 expression represents a novel mechanism of regulation of cellular gene expression by viral proteins. By targeting a ubiquitous second messenger such as calcium, HTLV-1 p12I may regulate the expression of the cellular transcriptional co-activator p300 to modulate viral gene expression and promote lymphocyte survival.
Figures
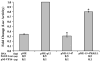
Similar articles
-
Human T lymphotropic virus type 1 accessory protein p12I modulates calcium-mediated cellular gene expression and enhances p300 expression in T lymphocytes.AIDS Res Hum Retroviruses. 2005 Apr;21(4):273-84. doi: 10.1089/aid.2005.21.273. AIDS Res Hum Retroviruses. 2005. PMID: 15943569 Free PMC article.
-
Histone acetyltransferase (HAT) activity of p300 modulates human T lymphotropic virus type 1 p30II-mediated repression of LTR transcriptional activity.Virology. 2006 Oct 25;354(2):225-39. doi: 10.1016/j.virol.2006.07.002. Epub 2006 Aug 4. Virology. 2006. PMID: 16890266 Free PMC article.
-
Role of accessory proteins of HTLV-1 in viral replication, T cell activation, and cellular gene expression.Front Biosci. 2004 Sep 1;9:2556-76. doi: 10.2741/1417. Front Biosci. 2004. PMID: 15358581 Free PMC article. Review.
-
Human T-cell lymphotropic virus type 1 p12I enhances interleukin-2 production during T-cell activation.J Virol. 2003 Oct;77(20):11027-39. doi: 10.1128/jvi.77.20.11027-11039.2003. J Virol. 2003. PMID: 14512551 Free PMC article.
-
Critical role of human T-lymphotropic virus type 1 accessory proteins in viral replication and pathogenesis.Microbiol Mol Biol Rev. 2002 Sep;66(3):396-406, table of contents. doi: 10.1128/MMBR.66.3.396-406.2002. Microbiol Mol Biol Rev. 2002. PMID: 12208996 Free PMC article. Review.
Cited by
-
Endoplasmic reticulum & mitochondrial calcium homeostasis: The interplay with viruses.Mitochondrion. 2021 May;58:227-242. doi: 10.1016/j.mito.2021.03.008. Epub 2021 Mar 26. Mitochondrion. 2021. PMID: 33775873 Free PMC article.
-
Orf-I and orf-II-encoded proteins in HTLV-1 infection and persistence.Viruses. 2011 Jun;3(6):861-85. doi: 10.3390/v3060861. Epub 2011 Jun 17. Viruses. 2011. PMID: 21994758 Free PMC article. Review.
-
Viral calciomics: interplays between Ca2+ and virus.Cell Calcium. 2009 Jul;46(1):1-17. doi: 10.1016/j.ceca.2009.05.005. Epub 2009 Jun 16. Cell Calcium. 2009. PMID: 19535138 Free PMC article. Review.
-
Calcium Ions Signaling: Targets for Attack and Utilization by Viruses.Front Microbiol. 2022 Jul 4;13:889374. doi: 10.3389/fmicb.2022.889374. eCollection 2022. Front Microbiol. 2022. PMID: 35859744 Free PMC article. Review.
-
Role of HTLV-1 orf-I encoded proteins in viral transmission and persistence.Retrovirology. 2019 Dec 18;16(1):43. doi: 10.1186/s12977-019-0502-1. Retrovirology. 2019. PMID: 31852543 Free PMC article. Review.
References
-
- Albrecht B, Collins ND, Newbound GC, Ratner L, Lairmore MD. Quantification of human T-cell lymphotropic virus type 1 proviral load by quantitative competitive polymerase chain reaction. J Virol Methods. 1998;75:123–140. - PubMed
-
- Ali SH, Decaprio JA. Cellular transformation by SV40 large T antigen: interaction with host proteins. Semin Cancer Biol. 2001;11:15–23. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

