Ribosomal protein S6 associates with alphavirus nonstructural protein 2 and mediates expression from alphavirus messages
- PMID: 16840351
- PMCID: PMC1563697
- DOI: 10.1128/JVI.00425-06
Ribosomal protein S6 associates with alphavirus nonstructural protein 2 and mediates expression from alphavirus messages
Abstract
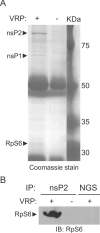
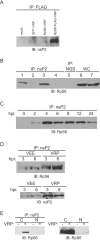
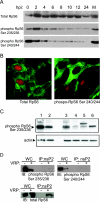
Although alphaviruses dramatically alter cellular function within hours of infection, interactions between alphaviruses and specific host cellular proteins are poorly understood. Although the alphavirus nonstructural protein 2 (nsP2) is an essential component of the viral replication complex, it also has critical auxiliary functions that determine the outcome of infection in the host. To gain a better understanding of nsP2 function, we sought to identify cellular proteins with which Venezuelan equine encephalitis virus nsP2 interacted. We demonstrate here that nsP2 associates with ribosomal protein S6 (RpS6) and that nsP2 is present in the ribosome-containing fractions of a polysome gradient, suggesting that nsP2 associates with RpS6 in the context of the whole ribosome. This result was noteworthy, since viral replicase proteins have seldom been described in direct association with components of the ribosome. The association of RpS6 with nsP2 was detected throughout the course of infection, and neither the synthesis of the viral structural proteins nor the presence of the other nonstructural proteins was required for RpS6 interaction with nsP2. nsP1 also was associated with RpS6, but other nonstructural proteins were not. RpS6 phosphorylation was dramatically diminished within hours after infection with alphaviruses. Furthermore, a reduction in the level of RpS6 protein expression led to diminished expression from alphavirus subgenomic messages, whereas no dramatic diminution in cellular translation was observed. Taken together, these data suggest that alphaviruses alter the ribosome during infection and that this alteration may contribute to differential translation of host and viral messages.
Figures

Similar articles
-
Comparative Characterization of the Sindbis Virus Proteome from Mammalian and Invertebrate Hosts Identifies nsP2 as a Component of the Virion and Sorting Nexin 5 as a Significant Host Factor for Alphavirus Replication.J Virol. 2018 Jun 29;92(14):e00694-18. doi: 10.1128/JVI.00694-18. Print 2018 Jul 15. J Virol. 2018. PMID: 29743363 Free PMC article.
-
Sindbis Virus Infection Causes Cell Death by nsP2-Induced Transcriptional Shutoff or by nsP3-Dependent Translational Shutoff.J Virol. 2018 Nov 12;92(23):e01388-18. doi: 10.1128/JVI.01388-18. Print 2018 Dec 1. J Virol. 2018. PMID: 30232189 Free PMC article.
-
Expression of Alphavirus Nonstructural Protein 2 (nsP2) in Mosquito Cells Inhibits Viral RNA Replication in Both a Protease Activity-Dependent and -Independent Manner.Viruses. 2022 Jun 17;14(6):1327. doi: 10.3390/v14061327. Viruses. 2022. PMID: 35746799 Free PMC article.
-
Persistent infection and suppression of host response by alphaviruses.Arch Virol Suppl. 2004;(18):139-47. doi: 10.1007/978-3-7091-0572-6_12. Arch Virol Suppl. 2004. PMID: 15119769 Review.
-
Alphavirus Infection: Host Cell Shut-Off and Inhibition of Antiviral Responses.Viruses. 2016 Jun 11;8(6):166. doi: 10.3390/v8060166. Viruses. 2016. PMID: 27294951 Free PMC article. Review.
Cited by
-
The Interplay of Viral and Host Factors in Chikungunya Virus Infection: Targets for Antiviral Strategies.Viruses. 2018 May 30;10(6):294. doi: 10.3390/v10060294. Viruses. 2018. PMID: 29849008 Free PMC article. Review.
-
Venezuelan equine encephalitis virus disrupts STAT1 signaling by distinct mechanisms independent of host shutoff.J Virol. 2009 Oct;83(20):10571-81. doi: 10.1128/JVI.01041-09. Epub 2009 Aug 5. J Virol. 2009. PMID: 19656875 Free PMC article.
-
The Host DHX9 DExH-Box Helicase Is Recruited to Chikungunya Virus Replication Complexes for Optimal Genomic RNA Translation.J Virol. 2019 Feb 5;93(4):e01764-18. doi: 10.1128/JVI.01764-18. Print 2019 Feb 15. J Virol. 2019. PMID: 30463980 Free PMC article.
-
Members of the ribosomal protein S6 (RPS6) family act as pro-viral factor for tomato spotted wilt orthotospovirus infectivity in Nicotiana benthamiana.Mol Plant Pathol. 2022 Mar;23(3):431-446. doi: 10.1111/mpp.13169. Epub 2021 Dec 16. Mol Plant Pathol. 2022. PMID: 34913556 Free PMC article.
-
Venezuelan Equine Encephalitis Virus Capsid-The Clever Caper.Viruses. 2017 Sep 29;9(10):279. doi: 10.3390/v9100279. Viruses. 2017. PMID: 28961161 Free PMC article. Review.
References
-
- Borchers, C., J. F. Peter, M. C. Hall, T. A. Kunkel, and K. B. Tomer. 2000. Identification of in-gel digested proteins by complementary peptide-mass fingerprinting and tandem mass spectrometry data obtained on an electrospray ionization quadrupole time-of-flight mass spectrometer. Anal. Chem. 72:1163-1168. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

