Kinetics of influenza A virus infection in humans
- PMID: 16840338
- PMCID: PMC1563736
- DOI: 10.1128/JVI.01623-05
Kinetics of influenza A virus infection in humans
Abstract
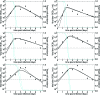
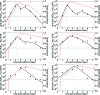
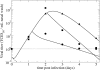
Currently, little is known about the viral kinetics of influenza A during infection within an individual. We utilize a series of mathematical models of increasing complexity, which incorporate target cell limitation and the innate interferon response, to examine influenza A virus kinetics in the upper respiratory tracts of experimentally infected adults. The models were fit to data from an experimental H1N1 influenza A/Hong Kong/123/77 infection and suggest that it is important to include the eclipse phase of the viral life cycle in viral dynamic models. Doing so, we estimate that after a delay of approximately 6 h, infected cells begin producing influenza virus and continue to do so for approximately 5 h. The average lifetime of infected cells is approximately 11 h, and the half-life of free infectious virus is approximately 3 h. We calculated the basic reproductive number, R(0), which indicated that a single infected cell could produce approximately 22 new productive infections. This suggests that antiviral treatments have a large hurdle to overcome in moderating symptoms and limiting infectiousness and that treatment has to be initiated as early as possible. For about 50% of patients, the curve of viral titer versus time has two peaks. This bimodal behavior can be explained by incorporating the antiviral effects of interferon into the model. Our model also compared well to an additional data set on viral titer after experimental infection and treatment with the neuraminidase inhibitor zanamivir, which suggests that such models may prove useful in estimating the efficacies of different antiviral therapies for influenza A infection.
Figures
Similar articles
-
Efficacy and safety of the neuraminidase inhibitor zanamivir in the treatment of influenzavirus infections. GG167 Influenza Study Group.N Engl J Med. 1997 Sep 25;337(13):874-80. doi: 10.1056/NEJM199709253371302. N Engl J Med. 1997. PMID: 9302301 Clinical Trial.
-
Oseltamivir resistance in influenza A (H5N1) infection.N Engl J Med. 2006 Mar 30;354(13):1423-4; author reply 1423-4. doi: 10.1056/NEJMc060077. N Engl J Med. 2006. PMID: 16571890 No abstract available.
-
Antiviral drugs in influenza: an adjunct to vaccination in some situations.Prescrire Int. 2006 Feb;15(81):21-30. Prescrire Int. 2006. PMID: 16548114
-
Influenza virus neuraminidase inhibitors.Lancet. 2000 Mar 4;355(9206):827-35. doi: 10.1016/S0140-6736(99)11433-8. Lancet. 2000. PMID: 10711940 Review.
-
Zanamivir: from drug design to the clinic.Philos Trans R Soc Lond B Biol Sci. 2001 Dec 29;356(1416):1885-93. doi: 10.1098/rstb.2001.1021. Philos Trans R Soc Lond B Biol Sci. 2001. PMID: 11779388 Free PMC article. Review.
Cited by
-
Kinetic model of development of acute viral infection in the human body. Critical conditions, control mechanisms, "thermoheliox".Russ Chem Bull. 2020;69(6):1179-1184. doi: 10.1007/s11172-020-2886-4. Epub 2020 Jul 20. Russ Chem Bull. 2020. PMID: 32834708 Free PMC article.
-
Transmission of a 2009 H1N1 pandemic influenza virus occurs before fever is detected, in the ferret model.PLoS One. 2012;7(8):e43303. doi: 10.1371/journal.pone.0043303. Epub 2012 Aug 29. PLoS One. 2012. PMID: 22952661 Free PMC article.
-
Compartmental Model Suggests Importance of Innate Immune Response to COVID-19 Infection in Rhesus Macaques.Bull Math Biol. 2021 May 26;83(7):79. doi: 10.1007/s11538-021-00909-0. Bull Math Biol. 2021. PMID: 34037874 Free PMC article.
-
Quantification of the Tradeoff between Test Sensitivity and Test Frequency in a COVID-19 Epidemic-A Multi-Scale Modeling Approach.Viruses. 2021 Mar 11;13(3):457. doi: 10.3390/v13030457. Viruses. 2021. PMID: 33799660 Free PMC article.
-
Identifying viral parameters from in vitro cell cultures.Front Microbiol. 2012 Sep 4;3:319. doi: 10.3389/fmicb.2012.00319. eCollection 2012. Front Microbiol. 2012. PMID: 22969758 Free PMC article.
References
-
- Beauchemin, C., J. Samuel, and J. Tuszynski. 2005. A simple cellular automaton model for influenza A viral infections. J. Theor. Biol. 232:223-234. - PubMed
-
- Bocharov, G. A., and A. A. Romanyukha. 1994. Mathematical model of antiviral immune response. III. Influenza A virus infection. J. Theor. Biol. 167:323-360. - PubMed
-
- Cella, M., F. Facchetti, A. Lanzavecchia, and M. Colonna. 2000. Plasmacytoid dendritic cells activated by influenza virus and CD40L drive a potent TH1 polarization. Nat. Immunol. 1:305-310. - PubMed
-
- Clarke, S. 1983. Physical defenses of the respiratory tract. Eur. J. Respir. Dis. Suppl. 126:27-30.
-
- Reference deleted.
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

