Serial passage through human glioma xenografts selects for a Deltagamma134.5 herpes simplex virus type 1 mutant that exhibits decreased neurotoxicity and prolongs survival of mice with experimental brain tumors
- PMID: 16840311
- PMCID: PMC1563698
- DOI: 10.1128/JVI.00725-06
Serial passage through human glioma xenografts selects for a Deltagamma134.5 herpes simplex virus type 1 mutant that exhibits decreased neurotoxicity and prolongs survival of mice with experimental brain tumors
Abstract
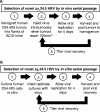
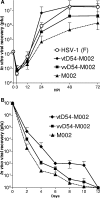
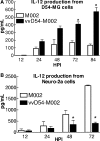
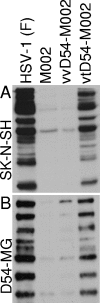
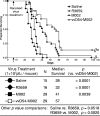
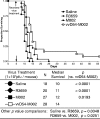
Previous studies have described in vitro serial passage of a Deltagamma(1)34.5 herpes simplex virus type 1 (HSV-1) strain in SK-N-SH neuroblastoma cells and selection of mutants that have acquired the ability to infect and replicate in this previously nonpermissive cell line. Here we describe the selection of a mutant HSV-1 strain by in vivo serial passage, which prolongs survival in two separate experimental murine brain tumor models. Two conditionally replication-competent Deltagamma(1)34.5 viruses, M002, which expresses murine interleukin-12, and its parent virus, R3659, were serially passaged within human malignant glioma D54-MG cell lines in vitro or flank tumor xenografts in vivo. The major findings are (i) viruses passaged in vivo demonstrate decreased neurovirulence, whereas those passaged in vitro demonstrate a partial recovery of the neurovirulence associated with HSV-1; and (ii) vvD54-M002, the virus selected after in vivo serial passage of M002 in D54-MG tumors, improves survival in two independent murine brain tumor models compared to the parent (unpassaged) M002. Additionally, in vitro-passaged, but not in vivo-passaged, M002 displayed changes in the protein synthesis profile in previously nonpermissive cell lines, as well as early U(S)11 transcription. Thus, a mutant HSV-1 strain expressing a foreign gene can be selected for enhanced antitumor efficacy via in vivo serial passage within flank D54-MG tumor xenografts. The enhanced antitumor efficacy of vvD54-M002 is not due to restoration of protein synthesis or early U(S)11 expression. This finding emphasizes the contribution of the in vivo tumor environment for selecting novel oncolytic HSV specifically adapted for tumor cell destruction in vivo.
Figures

Similar articles
-
Engineered herpes simplex virus expressing IL-12 in the treatment of experimental murine brain tumors.Proc Natl Acad Sci U S A. 2000 Feb 29;97(5):2208-13. doi: 10.1073/pnas.040557897. Proc Natl Acad Sci U S A. 2000. PMID: 10681459 Free PMC article.
-
Evaluation of genetically engineered herpes simplex viruses as oncolytic agents for human malignant brain tumors.Cancer Res. 1997 Apr 15;57(8):1502-9. Cancer Res. 1997. PMID: 9108452
-
Enhanced antiglioma activity of chimeric HCMV/HSV-1 oncolytic viruses.Gene Ther. 2007 Jul;14(13):1045-54. doi: 10.1038/sj.gt.3302942. Epub 2007 Apr 12. Gene Ther. 2007. PMID: 17429445
-
Active immunotherapy: oncolytic virus therapy using HSV-1.Adv Exp Med Biol. 2012;746:178-86. doi: 10.1007/978-1-4614-3146-6_14. Adv Exp Med Biol. 2012. PMID: 22639168 Review.
-
Genetically engineered human herpes simplex virus in the treatment of brain tumours.Herpes. 2001 Mar;8(1):17-22. Herpes. 2001. PMID: 11867012 Review.
Cited by
-
Preclinical evaluation of oncolytic δγ(1)34.5 herpes simplex virus expressing interleukin-12 for therapy of breast cancer brain metastases.Int J Breast Cancer. 2012;2012:628697. doi: 10.1155/2012/628697. Epub 2012 Dec 31. Int J Breast Cancer. 2012. PMID: 23346408 Free PMC article.
-
"Armed" oncolytic herpes simplex viruses for brain tumor therapy.Cell Adh Migr. 2008 Jul-Sep;2(3):208-13. doi: 10.4161/cam.2.3.6353. Epub 2008 Jul 28. Cell Adh Migr. 2008. PMID: 19262110 Free PMC article.
-
Intravesical treatment of advanced urothelial bladder cancers with oncolytic HSV-1 co-regulated by differentially expressed microRNAs.Gene Ther. 2016 May;23(5):460-8. doi: 10.1038/gt.2016.18. Epub 2016 Feb 23. Gene Ther. 2016. PMID: 26905370
-
Preclinical evaluation of a genetically engineered herpes simplex virus expressing interleukin-12.J Virol. 2012 May;86(9):5304-13. doi: 10.1128/JVI.06998-11. Epub 2012 Feb 29. J Virol. 2012. PMID: 22379082 Free PMC article.
-
Regression of advanced rat and human gliomas by local or systemic treatment with oncolytic parvovirus H-1 in rat models.Neuro Oncol. 2010 Aug;12(8):804-14. doi: 10.1093/neuonc/noq023. Epub 2010 Mar 18. Neuro Oncol. 2010. PMID: 20299703 Free PMC article.
References
-
- Andreansky, S., B. He, J. van Cott, J. McGhee, J. M. Markert, G. Y. Gillespie, B. Roizman, and R. J. Whitley. 1998. Treatment of intracranial gliomas in immunocompetent mice using herpes simplex viruses that express murine interleukins. Gene Ther. 5:121-130. - PubMed
-
- Andreansky, S., L. Soroceanu, E. R. Flotte, J. Chou, J. M. Markert, G. Y. Gillespie, B. Roizman, and R. J. Whitley. 1997. Evaluation of genetically engineered herpes simplex viruses as oncolytic agents for human malignant brain tumors. Cancer Res. 57:1502-1509. - PubMed
-
- Cassady, K. A., M. Gross, G. Y. Gillespie, and B. Roizman. 2002. Second-site mutation outside of the US10-12 domain of Δγ134.5 herpes simplex virus 1 recombinant blocks the shutoff of protein synthesis induced by activated protein kinase R and partially restores neurovirulence. J. Virol. 76:942-949. - PMC - PubMed
-
- Cassady, K. A., M. Gross, and B. Roizman. 1998. The herpes simplex virus US11 protein effectively compensates for the γ134.5 gene if present before activation of protein kinase R by precluding its phosphorylation and that of the α subunit of eukaryotic translation initiation factor 2. J. Virol. 72:8620-8626. - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

