Mitochondrial pathology and muscle and dopaminergic neuron degeneration caused by inactivation of Drosophila Pink1 is rescued by Parkin
- PMID: 16818890
- PMCID: PMC1502310
- DOI: 10.1073/pnas.0602493103
Mitochondrial pathology and muscle and dopaminergic neuron degeneration caused by inactivation of Drosophila Pink1 is rescued by Parkin
Abstract
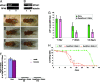
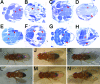
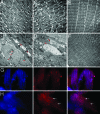
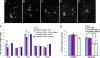
Mutations in Pink1, a gene encoding a Ser/Thr kinase with a mitochondrial-targeting signal, are associated with Parkinson's disease (PD), the most common movement disorder characterized by selective loss of dopaminergic neurons. The mechanism by which loss of Pink1 leads to neurodegeneration is not understood. Here we show that inhibition of Drosophila Pink1 (dPink1) function results in energy depletion, shortened lifespan, and degeneration of select indirect flight muscles and dopaminergic neurons. The muscle pathology was preceded by mitochondrial enlargement and disintegration. These phenotypes could be rescued by the wild type but not the pathogenic C-terminal deleted form of human Pink1 (hPink1). The muscle and dopaminergic phenotypes associated with dPink1 inactivation show similarity to that seen in parkin mutant flies and could be suppressed by the overexpression of Parkin but not DJ-1. Consistent with the genetic rescue results, we find that, in dPink1 RNA interference (RNAi) animals, the level of Parkin protein is significantly reduced. Together, these results implicate Pink1 and Parkin in a common pathway that regulates mitochondrial physiology and cell survival in Drosophila.
Conflict of interest statement
Conflict of interest statement: No conflicts declared.
Figures

Similar articles
-
Drosophila pink1 is required for mitochondrial function and interacts genetically with parkin.Nature. 2006 Jun 29;441(7097):1162-6. doi: 10.1038/nature04779. Epub 2006 May 3. Nature. 2006. PMID: 16672981
-
Mitochondrial dysfunction in Drosophila PINK1 mutants is complemented by parkin.Nature. 2006 Jun 29;441(7097):1157-61. doi: 10.1038/nature04788. Epub 2006 May 3. Nature. 2006. PMID: 16672980
-
The loss of PGAM5 suppresses the mitochondrial degeneration caused by inactivation of PINK1 in Drosophila.PLoS Genet. 2010 Dec 2;6(12):e1001229. doi: 10.1371/journal.pgen.1001229. PLoS Genet. 2010. PMID: 21151955 Free PMC article.
-
PINK1-Parkin signaling in Parkinson's disease: Lessons from Drosophila.Neurosci Res. 2020 Oct;159:40-46. doi: 10.1016/j.neures.2020.01.016. Epub 2020 Feb 6. Neurosci Res. 2020. PMID: 32035987 Review.
-
Parkin blushed by PINK1.Neuron. 2006 May 18;50(4):527-9. doi: 10.1016/j.neuron.2006.05.003. Neuron. 2006. PMID: 16701203 Review.
Cited by
-
The p53 target DRAM1 modulates calcium homeostasis and ER stress by promoting contact between lysosomes and the ER through STIM1.Proc Natl Acad Sci U S A. 2024 Sep 24;121(39):e2400531121. doi: 10.1073/pnas.2400531121. Epub 2024 Sep 18. Proc Natl Acad Sci U S A. 2024. PMID: 39292746
-
Mitochondrial quality control: a matter of life and death for neurons.EMBO J. 2012 Mar 21;31(6):1336-49. doi: 10.1038/emboj.2012.38. Epub 2012 Feb 21. EMBO J. 2012. PMID: 22354038 Free PMC article. Review.
-
Loss of Mfn2 results in progressive, retrograde degeneration of dopaminergic neurons in the nigrostriatal circuit.Hum Mol Genet. 2012 Nov 15;21(22):4817-26. doi: 10.1093/hmg/dds311. Epub 2012 Jul 31. Hum Mol Genet. 2012. PMID: 22859504 Free PMC article.
-
Parkin-catalyzed ubiquitin-ester transfer is triggered by PINK1-dependent phosphorylation.J Biol Chem. 2013 Jul 26;288(30):22019-32. doi: 10.1074/jbc.M113.467530. Epub 2013 Jun 10. J Biol Chem. 2013. PMID: 23754282 Free PMC article.
-
Gut microbiome and Parkinson's disease: Perspective on pathogenesis and treatment.J Adv Res. 2023 Aug;50:83-105. doi: 10.1016/j.jare.2022.10.013. Epub 2022 Nov 1. J Adv Res. 2023. PMID: 36332796 Free PMC article. Review.
References
-
- Dunnett S. B., Bjorklund A. Nature. 1999;399:A32–A39. - PubMed
-
- Dawson T. M., Dawson V. L. Science. 2003;302:819–822. - PubMed
-
- Bertoli-Avella A. M., Oostra B. A., Heutink P. Hum. Genet. 2004;114:413–438. - PubMed
-
- Polymeropoulos M. H., Lavedan C., Leroy E., Ide S. E., Dehejia A., Dutra A., Pike B., Root H., Rubenstein J., Boyer R., et al. Science. 1997;276:2045–2047. - PubMed
-
- Kitada T., Asakawa S., Hattori N., Matsumine H., Yamamura Y., Minoshima S., Yokochi M., Mizuno Y., Shimizu N. Nature. 1998;392:605–608. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous

