Direct interaction of focal adhesion kinase (FAK) with Met is required for FAK to promote hepatocyte growth factor-induced cell invasion
- PMID: 16782899
- PMCID: PMC1489146
- DOI: 10.1128/MCB.02186-05
Direct interaction of focal adhesion kinase (FAK) with Met is required for FAK to promote hepatocyte growth factor-induced cell invasion
Abstract
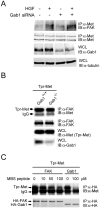
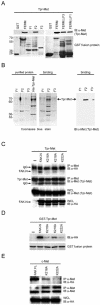
Focal adhesion kinase (FAK) has been implicated to be a point of convergence of integrin and growth factor signaling pathways. Here we report that FAK directly interacts with the hepatocyte growth factor receptor c-Met. Phosphorylation of c-Met at Tyr-1349 and, to a lesser extent, Tyr-1356 is required for its interaction with the band 4.1 and ezrin/radixin/moesin homology domain (FERM domain) of FAK. The F2 subdomain of the FAK FERM domain alone is sufficient for Met binding, in which a patch of basic residues (216KAKTLRK222) are critical for the interaction. Met-FAK interaction leads to FAK activation and subsequent contribution to hepatocyte growth factor-induced cell motility and cell invasion. Our results provide evidence that constitutive Met-FAK interaction may be a critical determinant for tumor cells to acquire invasive potential.
Figures
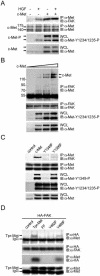

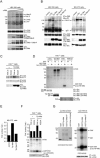


 , P < 0.05, compared with the control MDCK cells. (C) The MDCK cells were subjected to a cell migration assay.
, P < 0.05, compared with the control MDCK cells. (C) The MDCK cells were subjected to a cell migration assay.  , P < 0.05, compared with the control MDCK cells in the presence of HGF. (D) The MDCK cells were subjected to a Matrigel invasion assay.
, P < 0.05, compared with the control MDCK cells in the presence of HGF. (D) The MDCK cells were subjected to a Matrigel invasion assay.  , P < 0.05, compared with the control MDCK cells in the presence of HGF. (E) Met-FAK interaction was analyzed with a series of lung cancer cell lines. The ability of those cells to invade through Matrigel was measured.
, P < 0.05, compared with the control MDCK cells in the presence of HGF. (E) Met-FAK interaction was analyzed with a series of lung cancer cell lines. The ability of those cells to invade through Matrigel was measured.  , P < 0.05, compared with A549 cells. (F) CL1-5 lung cancer cells stably expressing the FAK NH2 domain were established. The inhibitory effect of the FAK NH2 domain on Met-FAK interaction was examined. (G) CL1-5 cells and those stably expressing the FAK NH2 domain were subjected to a Matrigel invasion assay. IP, immunoprecipitation; WCL, whole-cell lysates; IgG, immunoglobulin G.
, P < 0.05, compared with A549 cells. (F) CL1-5 lung cancer cells stably expressing the FAK NH2 domain were established. The inhibitory effect of the FAK NH2 domain on Met-FAK interaction was examined. (G) CL1-5 cells and those stably expressing the FAK NH2 domain were subjected to a Matrigel invasion assay. IP, immunoprecipitation; WCL, whole-cell lysates; IgG, immunoglobulin G.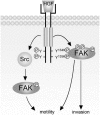
Similar articles
-
Phosphorylation of focal adhesion kinase on tyrosine 194 by Met leads to its activation through relief of autoinhibition.Oncogene. 2011 Jan 13;30(2):153-66. doi: 10.1038/onc.2010.398. Epub 2010 Aug 30. Oncogene. 2011. PMID: 20802513
-
Adaptor molecule Crk is required for sustained phosphorylation of Grb2-associated binder 1 and hepatocyte growth factor-induced cell motility of human synovial sarcoma cell lines.Mol Cancer Res. 2006 Jul;4(7):499-510. doi: 10.1158/1541-7786.MCR-05-0141. Mol Cancer Res. 2006. PMID: 16849525
-
Src and FAK mediate cell-matrix adhesion-dependent activation of Met during transformation of breast epithelial cells.J Cell Biochem. 2009 Aug 15;107(6):1168-81. doi: 10.1002/jcb.22219. J Cell Biochem. 2009. PMID: 19533669
-
Signal transduction by focal adhesion kinase in cancer.Cancer Metastasis Rev. 2009 Jun;28(1-2):35-49. doi: 10.1007/s10555-008-9165-4. Cancer Metastasis Rev. 2009. PMID: 19169797 Review.
-
New concepts regarding focal adhesion kinase promotion of cell migration and proliferation.J Cell Biochem. 2006 Sep 1;99(1):35-52. doi: 10.1002/jcb.20956. J Cell Biochem. 2006. PMID: 16823799 Review.
Cited by
-
Genome-wide analysis of Musashi-2 targets reveals novel functions in governing epithelial cell migration.Nucleic Acids Res. 2016 May 5;44(8):3788-800. doi: 10.1093/nar/gkw207. Epub 2016 Mar 31. Nucleic Acids Res. 2016. PMID: 27034466 Free PMC article.
-
MicroRNA-34a modulates c-Myc transcriptional complexes to suppress malignancy in human prostate cancer cells.PLoS One. 2012;7(1):e29722. doi: 10.1371/journal.pone.0029722. Epub 2012 Jan 3. PLoS One. 2012. PMID: 22235332 Free PMC article.
-
Aberrant MET Receptor Tyrosine Kinase Signaling in Glioblastoma: Targeted Therapy and Future Directions.Cells. 2024 Jan 25;13(3):218. doi: 10.3390/cells13030218. Cells. 2024. PMID: 38334610 Free PMC article. Review.
-
Recycling machinery of integrin coupled with focal adhesion turnover via RAB11-UNC13D-FAK axis for migration of pancreatic cancer cells.J Transl Med. 2024 Aug 29;22(1):800. doi: 10.1186/s12967-024-05630-9. J Transl Med. 2024. PMID: 39210440 Free PMC article.
-
Focal adhesion kinase regulation of mechanotransduction and its impact on endothelial cell functions.Microvasc Res. 2012 Jan;83(1):71-81. doi: 10.1016/j.mvr.2011.06.007. Epub 2011 Jun 29. Microvasc Res. 2012. PMID: 21741394 Free PMC article. Review.
References
-
- Bardelli, A., L. Pugliese, and P. M. Comoglio. 1997. “Invasive-growth” signaling by the Met/HGF receptor: the hereditary renal carcinoma connection. Biochim. Biophys. Acta 1333:M41-M51. - PubMed
-
- Birchmeier, C., W. Birchmeier, E. Gherardi, and G. F. Vande Woude. 2003. Met, metastasis, motility and more. Nat. Rev. Mol. Cell Biol. 4:915-925. - PubMed
-
- Birchmeier, C., and E. Gherardi. 1998. Developmental roles of HGF/SF and its receptor, the c-Met tyrosine kinase. Trends Cell Biol. 8:404-410. - PubMed
-
- Bottaro, D. P., J. S. Rubin, D. L. Faletto, A. M. Chan, T. E. Kmiecik, G. F. Vande Woude, and S. A. Aaronson. 1991. Identification of the hepatocyte growth factor receptor as the c-met proto-oncogene product. Science 251:802-804. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials
Miscellaneous
