Regulation of nitrogenase by 2-oxoglutarate-reversible, direct binding of a PII-like nitrogen sensor protein to dinitrogenase
- PMID: 16777963
- PMCID: PMC1502530
- DOI: 10.1073/pnas.0602278103
Regulation of nitrogenase by 2-oxoglutarate-reversible, direct binding of a PII-like nitrogen sensor protein to dinitrogenase
Abstract
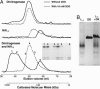
Posttranslational regulation of nitrogenase, or switch-off, in the methanogenic archaeon Methanococcus maripaludis requires both nifI(1) and nifI(2), which encode members of the PII family of nitrogen-regulatory proteins. Previous work demonstrated that nitrogenase activity in cell extracts was inhibited in the presence of NifI(1) and NifI(2), and that 2-oxoglutarate (2OG), a potential signal of nitrogen limitation, relieved this inhibition. To further explore the role of the NifI proteins in switch-off, we found proteins that interact with NifI(1) and NifI(2) and determined whether 2OG affected these interactions. Anaerobic purification of His-tagged NifI(2) resulted in copurification of NifI(1) and the dinitrogenase subunits NifD and NifK, and 2OG or a deletion mutation affecting the T-loop of NifI(2) prevented copurification of dinitrogenase but did not affect copurification of NifI(1). Similar results were obtained with His-tagged NifI(1). Gel-filtration chromatography demonstrated an interaction between purified NifI(1,2) and dinitrogenase that was inhibited by 2OG. The NifI proteins themselves formed a complex of approximately 85 kDa, which appeared to further oligomerize in the presence of 2OG. NifI(1,2) inhibited activity of purified nitrogenase when present in a 1:1 molar ratio to dinitrogenase, and 2OG fully relieved this inhibition. These results suggest a model for switch-off of nitrogenase activity, where direct interaction of a NifI(1,2) complex with dinitrogenase causes inhibition, which is relieved by 2OG. The presence of nifI(1) and nifI(2) in the nif operons of all nitrogen-fixing Archaea and some anaerobic Bacteria suggests that this mode of nitrogenase regulation may operate in a wide variety of diazotrophs.
Figures
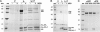

Similar articles
-
2-Oxoglutarate and the PII homologues NifI1 and NifI2 regulate nitrogenase activity in cell extracts of Methanococcus maripaludis.Mol Microbiol. 2005 Jun;56(6):1527-38. doi: 10.1111/j.1365-2958.2005.04621.x. Mol Microbiol. 2005. PMID: 15916603
-
NifI inhibits nitrogenase by competing with Fe protein for binding to the MoFe protein.Biochem Biophys Res Commun. 2007 Dec 14;364(2):378-82. doi: 10.1016/j.bbrc.2007.10.020. Epub 2007 Oct 15. Biochem Biophys Res Commun. 2007. PMID: 17950693
-
Ammonia switch-off of nitrogen fixation in the methanogenic archaeon Methanococcus maripaludis: mechanistic features and requirement for the novel GlnB homologues, NifI(1) and NifI(2).J Bacteriol. 2001 Feb;183(3):882-9. doi: 10.1128/JB.183.3.882-889.2001. J Bacteriol. 2001. PMID: 11208785 Free PMC article.
-
Nitrogen regulation in bacteria and archaea.Annu Rev Microbiol. 2007;61:349-77. doi: 10.1146/annurev.micro.61.080706.093409. Annu Rev Microbiol. 2007. PMID: 17506680 Review.
-
Regulation of nitrogen fixation in Klebsiella pneumoniae and Azotobacter vinelandii: NifL, transducing two environmental signals to the nif transcriptional activator NifA.J Mol Microbiol Biotechnol. 2002 May;4(3):235-42. J Mol Microbiol Biotechnol. 2002. PMID: 11931553 Review.
Cited by
-
H2-independent growth of the hydrogenotrophic methanogen Methanococcus maripaludis.mBio. 2013 Feb 26;4(2):e00062-13. doi: 10.1128/mBio.00062-13. mBio. 2013. PMID: 23443005 Free PMC article.
-
Effects of H2 and formate on growth yield and regulation of methanogenesis in Methanococcus maripaludis.J Bacteriol. 2013 Apr;195(7):1456-62. doi: 10.1128/JB.02141-12. Epub 2013 Jan 18. J Bacteriol. 2013. PMID: 23335420 Free PMC article.
-
Protein complexing in a methanogen suggests electron bifurcation and electron delivery from formate to heterodisulfide reductase.Proc Natl Acad Sci U S A. 2010 Jun 15;107(24):11050-5. doi: 10.1073/pnas.1003653107. Epub 2010 Jun 1. Proc Natl Acad Sci U S A. 2010. PMID: 20534465 Free PMC article.
-
Global responses of Methanococcus maripaludis to specific nutrient limitations and growth rate.J Bacteriol. 2008 Mar;190(6):2198-205. doi: 10.1128/JB.01805-07. Epub 2008 Jan 18. J Bacteriol. 2008. PMID: 18203827 Free PMC article.
-
Efficient CRISPR/Cas12a-Based Genome-Editing Toolbox for Metabolic Engineering in Methanococcus maripaludis.ACS Synth Biol. 2022 Jul 15;11(7):2496-2503. doi: 10.1021/acssynbio.2c00137. Epub 2022 Jun 22. ACS Synth Biol. 2022. PMID: 35730587 Free PMC article.
References
-
- Halbleib C. M., Ludden P. W. J. Nutr. 2000;130:1081–1084. - PubMed
-
- Rees D. C., Tezcan F. A., Haynes C. A., Walton M. Y., Andrade S., Einsle O., Howard J. B. Philos. Trans. R. Soc. London A. 2005;363:971–984. - PubMed
-
- Ludden P. W., Roberts G. P. Curr. Top. Cell. Regul. 1989;30:23–56. - PubMed
-
- Yoch D. C., Li J. D., Hu C. Z., Scholin C. Arch. Microbiol. 1988;150:1–5. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

