Immunogenicity of cytopathic and noncytopathic viral vectors
- PMID: 16775313
- PMCID: PMC1488949
- DOI: 10.1128/JVI.00084-06
Immunogenicity of cytopathic and noncytopathic viral vectors
Abstract
The impact of cytolytic versus noncytolytic viral infections on host responses is not well understood, due to limitations of the systems that have been used to address this issue. Using paired cytopathic and noncytopathic rabies viruses that differ by only two amino acids, we investigated several fundamental aspects of the immune response to these viral vectors. Greater cytopathic capacity translated into a greater degree of cross-priming to CD8(+) T cells (T(CD8)(+)) and more-robust short-term humoral and cellular responses. However, long-term responses to the two viruses were similar, suggesting that direct priming drives the bulk of the T(CD8)(+) antirabies response and that enhanced acute responses associated with greater virally mediated cellular destruction were balanced by other factors, such as prolonged antigen expression associated with noncytopathic virus. Such compensatory mechanisms may be in place to ensure comparable immunologic memories to various pathogens.
Figures

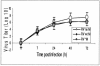
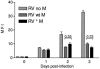

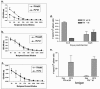
Similar articles
-
Antigenic and molecular characterization of field and vaccine rabies virus strains in the Russian Federation.Dev Biol (Basel). 2006;125:33-7. Dev Biol (Basel). 2006. PMID: 16878458 No abstract available.
-
Vaccination with a plasmid vector carrying the rabies virus glycoprotein gene induces protective immunity against rabies virus.Virology. 1994 Feb 15;199(1):132-40. doi: 10.1006/viro.1994.1105. Virology. 1994. PMID: 8116236
-
Canine adenoviruses elicit both humoral and cell-mediated immune responses against rabies following immunisation of sheep.Vaccine. 2011 Feb 1;29(6):1304-10. doi: 10.1016/j.vaccine.2010.11.068. Epub 2010 Dec 4. Vaccine. 2011. PMID: 21134446
-
Canine adenovirus based rabies vaccines.Dev Biol (Basel). 2008;131:467-76. Dev Biol (Basel). 2008. PMID: 18634509 Review.
-
Rabies vaccine: traditional and novel approaches.Prog Vet Microbiol Immunol. 1987;3:73-111. Prog Vet Microbiol Immunol. 1987. PMID: 3078836 Review. No abstract available.
Cited by
-
A role for granulocyte-macrophage colony-stimulating factor in the regulation of CD8(+) T cell responses to rabies virus.Virology. 2012 May 10;426(2):120-33. doi: 10.1016/j.virol.2012.01.025. Epub 2012 Feb 16. Virology. 2012. PMID: 22341782 Free PMC article.
-
A novel composite immunotoxin that suppresses rabies virus production by the infected cells.J Immunol Methods. 2010 Feb 28;353(1-2):78-86. doi: 10.1016/j.jim.2009.11.010. Epub 2009 Nov 22. J Immunol Methods. 2010. PMID: 19932697 Free PMC article.
-
Guanylyl cyclase C-induced immunotherapeutic responses opposing tumor metastases without autoimmunity.J Natl Cancer Inst. 2008 Jul 2;100(13):950-61. doi: 10.1093/jnci/djn178. Epub 2008 Jun 24. J Natl Cancer Inst. 2008. PMID: 18577748 Free PMC article.
-
Designing CD8+ T cell vaccines: it's not rocket science (yet).Curr Opin Immunol. 2010 Jun;22(3):402-10. doi: 10.1016/j.coi.2010.04.002. Epub 2010 May 4. Curr Opin Immunol. 2010. PMID: 20447814 Free PMC article. Review.
-
A Quasi-Steady-State Approximation to the Basic Target-Cell-Limited Viral Dynamics Model with a Non-Cytopathic Effect.Front Microbiol. 2018 Jan 31;9:54. doi: 10.3389/fmicb.2018.00054. eCollection 2018. Front Microbiol. 2018. PMID: 29445361 Free PMC article.
References
-
- Albert, M. L., B. Sauter, and N. Bhardwaj. 1998. Dendritic cells acquire antigen from apoptotic cells and induce class I-restricted CTLs. Nature 392:86-89. - PubMed
-
- Barr, J. N., S. P. Whelan, and G. W. Wertz. 2002. Transcriptional control of the RNA-dependent RNA polymerase of vesicular stomatitis virus. Biochim. Biophys. Acta 1577:337-353. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Research Materials

