Dual loss of ER export and endocytic signals with altered melanosome morphology in the silver mutation of Pmel17
- PMID: 16760433
- PMCID: PMC1525253
- DOI: 10.1091/mbc.e06-01-0081
Dual loss of ER export and endocytic signals with altered melanosome morphology in the silver mutation of Pmel17
Abstract
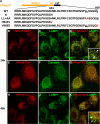
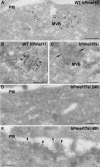
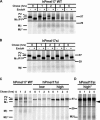
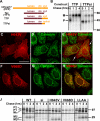
Pmel17 is a pigment cell-specific integral membrane protein that participates in the formation of the intralumenal fibrils upon which melanins are deposited in melanosomes. The Pmel17 cytoplasmic domain is truncated by the mouse silver mutation, which is associated with coat hypopigmentation in certain strain backgrounds. Here, we show that the truncation interferes with at least two steps in Pmel17 intracellular transport, resulting in defects in melanosome biogenesis. Human Pmel17 engineered with the truncation found in the mouse silver mutant (hPmel17si) is inefficiently exported from the endoplasmic reticulum (ER). Localization and metabolic pulse-chase analyses with site-directed mutants and chimeric proteins show that this effect is due to the loss of a conserved C-terminal valine that serves as an ER exit signal. hPmel17si that exits the ER accumulates abnormally at the plasma membrane due to the loss of a di-leucine-based endocytic signal. The combined effects of reduced ER export and endocytosis significantly deplete Pmel17 within endocytic compartments and delay proteolytic maturation required for premelanosome-like fibrillogenesis. The ER export delay and cell surface retention are also observed for endogenous Pmel17si in melanocytes from silver mice, within which Pmel17 accumulation in premelanosomes is dramatically reduced. Mature melanosomes in these cells are larger, rounder, more highly pigmented, and less striated than in control melanocytes. These data reveal a dual sorting defect in a natural mutant of Pmel17 and support a requirement of endocytic trafficking in Pmel17 fibril formation.
Figures



Similar articles
-
Endoplasmic reticulum export, subcellular distribution, and fibril formation by Pmel17 require an intact N-terminal domain junction.J Biol Chem. 2010 May 21;285(21):16166-83. doi: 10.1074/jbc.M109.097725. Epub 2010 Mar 15. J Biol Chem. 2010. PMID: 20231267 Free PMC article.
-
Premelanosome amyloid-like fibrils are composed of only golgi-processed forms of Pmel17 that have been proteolytically processed in endosomes.J Biol Chem. 2008 Jan 25;283(4):2307-22. doi: 10.1074/jbc.M708007200. Epub 2007 Nov 8. J Biol Chem. 2008. PMID: 17991747 Free PMC article.
-
N-terminal domains elicit formation of functional Pmel17 amyloid fibrils.J Biol Chem. 2009 Dec 18;284(51):35543-55. doi: 10.1074/jbc.M109.047449. J Biol Chem. 2009. PMID: 19840945 Free PMC article.
-
The Silver locus product Pmel17/gp100/Silv/ME20: controversial in name and in function.Pigment Cell Res. 2005 Oct;18(5):322-36. doi: 10.1111/j.1600-0749.2005.00269.x. Pigment Cell Res. 2005. PMID: 16162173 Free PMC article. Review.
-
Assembly, target-signaling and intracellular transport of tyrosinase gene family proteins in the initial stage of melanosome biogenesis.Pigment Cell Res. 2000 Aug;13(4):222-9. doi: 10.1034/j.1600-0749.2000.130403.x. Pigment Cell Res. 2000. PMID: 10952389 Review.
Cited by
-
Molecular and Biochemical Basis of Minocycline-Induced Hyperpigmentation-The Study on Normal Human Melanocytes Exposed to UVA and UVB Radiation.Int J Mol Sci. 2021 Apr 4;22(7):3755. doi: 10.3390/ijms22073755. Int J Mol Sci. 2021. PMID: 33916535 Free PMC article.
-
The Apaf-1-binding protein Aven is cleaved by Cathepsin D to unleash its anti-apoptotic potential.Cell Death Differ. 2012 Sep;19(9):1435-45. doi: 10.1038/cdd.2012.17. Epub 2012 Mar 2. Cell Death Differ. 2012. PMID: 22388353 Free PMC article.
-
A missense mutation in PMEL17 is associated with the Silver coat color in the horse.BMC Genet. 2006 Oct 9;7:46. doi: 10.1186/1471-2156-7-46. BMC Genet. 2006. PMID: 17029645 Free PMC article.
-
The secreted form of a melanocyte membrane-bound glycoprotein (Pmel17/gp100) is released by ectodomain shedding.FASEB J. 2010 Mar;24(3):916-30. doi: 10.1096/fj.09-140921. Epub 2009 Nov 2. FASEB J. 2010. PMID: 19884326 Free PMC article.
-
MHC class II presentation of gp100 epitopes in melanoma cells requires the function of conventional endosomes and is influenced by melanosomes.J Immunol. 2008 Dec 1;181(11):7843-52. doi: 10.4049/jimmunol.181.11.7843. J Immunol. 2008. PMID: 19017974 Free PMC article.
References
-
- Adema G. J., de Boer A. J., Vogel A. M., Loenen W. A. M., Figdor C. G. Molecular characterization of the melanocyte lineage-specific antigen gp100. J. Biol. Chem. 1994;269:20126–20133. - PubMed
-
- Basrur V., et al. Proteomic analysis of early melanosomes: identification of novel melanosomal proteins. J. Proteome Res. 2003;2:69–79. - PubMed
-
- Bennett D. C., Cooper P. J., Hart I. R. A line of non-tumorigenic mouse melanocytes, syngeneic with the B16 melanoma and requiring a tumour promoter for growth. Int. J. Cancer. 1987;39:414–418. - PubMed
-
- Bennett D. C., Lamoreux M. L. The color loci of mice—a genetic century. Pigment Cell Res. 2003;16:333–344. - PubMed
-
- Berson J. F., Frank D. W., Calvo P. A., Bieler B. M., Marks M. S. A common temperature-sensitive allelic form of human tyrosinase is retained in the endoplasmic reticulum at the nonpermissive temperature. J. Biol. Chem. 2000;275:12281–12289. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous

