Calcium-sensing receptor abrogates secretagogue- induced increases in intestinal net fluid secretion by enhancing cyclic nucleotide destruction
- PMID: 16760252
- PMCID: PMC1475505
- DOI: 10.1073/pnas.0602996103
Calcium-sensing receptor abrogates secretagogue- induced increases in intestinal net fluid secretion by enhancing cyclic nucleotide destruction
Abstract
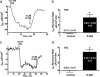
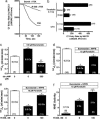
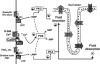
The calcium-sensing receptor (CaSR) provides a fundamental mechanism for diverse cells to detect and respond to modulations in the ionic and nutrient compositions of their extracellular milieu. The roles for this receptor are largely unknown in the intestinal tract, where epithelial cells are normally exposed to large variations in extracellular solutes. Here, we show that colonic CaSR signaling stimulates the degradation of cyclic nucleotides by phosphodiesterases and describe the ability of receptor activation to reverse the fluid and electrolyte secretory actions of cAMP- and cGMP-generating secretagogues, including cholera toxin and heat stable Escherichia coli enterotoxin STa. Our results suggest a paradigm for regulation of intestinal fluid transport where fine tuning is accomplished by the counterbalancing effects of solute activation of the CaSR on neuronal and hormonal secretagogue actions. The reversal of cholera toxin- and STa endotoxin-induced fluid secretion by a small-molecule CaSR agonist suggests that these compounds may provide a unique therapy for secretory diarrheas.
Conflict of interest statement
Conflict of interest statement: S.C.H. is an inventor on patents covering CaSR active molecules, receives royalties on calcimimetics, and is a consultant on calcimimetics at Amgen, Inc. This work was supported in part by a gift from Amgen.
Figures

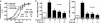


Comment in
-
Profile of Steven C. Hebert.Proc Natl Acad Sci U S A. 2006 Jun 20;103(25):9387-9. doi: 10.1073/pnas.0604149103. Epub 2006 Jun 13. Proc Natl Acad Sci U S A. 2006. PMID: 16772375 Free PMC article. No abstract available.
Similar articles
-
Calcium-sensing receptor inhibits secretagogue-induced electrolyte secretion by intestine via the enteric nervous system.Am J Physiol Gastrointest Liver Physiol. 2012 Jul;303(1):G60-70. doi: 10.1152/ajpgi.00425.2011. Epub 2012 Apr 19. Am J Physiol Gastrointest Liver Physiol. 2012. PMID: 22517767 Free PMC article.
-
Calcium-sensing receptor stimulates Cl(-)- and SCFA-dependent but inhibits cAMP-dependent HCO3(-) secretion in colon.Am J Physiol Gastrointest Liver Physiol. 2015 May 15;308(10):G874-83. doi: 10.1152/ajpgi.00341.2014. Epub 2015 Mar 19. Am J Physiol Gastrointest Liver Physiol. 2015. PMID: 25792563 Free PMC article.
-
A novel cation-sensing mechanism in osteoblasts is a molecular target for strontium.J Bone Miner Res. 2004 May;19(5):862-9. doi: 10.1359/JBMR.040114. Epub 2004 Jan 12. J Bone Miner Res. 2004. PMID: 15068510
-
Calcimimetics and hyperparathyroidism.Curr Opin Investig Drugs. 2004 Oct;5(10):1080-5. Curr Opin Investig Drugs. 2004. PMID: 15535429 Review.
-
Calcium-sensing receptor: A new target for therapy of diarrhea.World J Gastroenterol. 2016 Mar 7;22(9):2711-24. doi: 10.3748/wjg.v22.i9.2711. World J Gastroenterol. 2016. PMID: 26973410 Free PMC article. Review.
Cited by
-
The calcium-sensing receptor and its interacting proteins.J Cell Mol Med. 2007 Sep-Oct;11(5):923-34. doi: 10.1111/j.1582-4934.2007.00114.x. J Cell Mol Med. 2007. PMID: 17979874 Free PMC article. Review.
-
Mechanisms by which calcium receptor stimulation modifies electromechanical coupling in isolated ventricular cardiomyocytes.Pflugers Arch. 2015 Feb;467(2):379-88. doi: 10.1007/s00424-014-1498-y. Epub 2014 Apr 1. Pflugers Arch. 2015. PMID: 24687204
-
Epithelial CaSR deficiency alters intestinal integrity and promotes proinflammatory immune responses.FEBS Lett. 2014 Nov 17;588(22):4158-66. doi: 10.1016/j.febslet.2014.05.007. Epub 2014 May 17. FEBS Lett. 2014. PMID: 24842610 Free PMC article.
-
We may be able to stop common lethal secretory diarrhea by activating the intestinal calcium sensing receptors.MedGenMed. 2007 Jan 12;9(1):9. MedGenMed. 2007. PMID: 17435618 Free PMC article. No abstract available.
-
Calcimimetics in CKD-results from recent clinical studies.Pediatr Nephrol. 2008 Oct;23(10):1721-8. doi: 10.1007/s00467-008-0900-4. Epub 2008 Jul 2. Pediatr Nephrol. 2008. PMID: 18594867 Free PMC article. Review.
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

