An in-house RD1-based enzyme-linked immunospot-gamma interferon assay instead of the tuberculin skin test for diagnosis of latent Mycobacterium tuberculosis infection
- PMID: 16757583
- PMCID: PMC1489403
- DOI: 10.1128/JCM.02265-05
An in-house RD1-based enzyme-linked immunospot-gamma interferon assay instead of the tuberculin skin test for diagnosis of latent Mycobacterium tuberculosis infection
Abstract
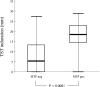
Identification of individuals infected with Mycobacterium tuberculosis is essential for the control of tuberculosis (TB). The specificity of the currently used tuberculin skin test (TST) is poor because of the broad antigenic cross-reactivity of purified protein derivative (PPD) with BCG vaccine strains and environmental mycobacteria. Both ESAT-6 and CFP-10, two secretory proteins that are highly specific for M. tuberculosis complex, elicit strong T-cell responses in subjects with TB. Using an enzyme-linked immunospot (ELISPOT)-IFN-gamma assay and a restricted pool of peptides derived from ESAT-6 and CFP-10, we have previously demonstrated a high degree of specificity and sensitivity of the test for the diagnosis of TB. Here, 119 contacts of individuals with contagious TB who underwent TST and the ELISPOT-IFN-gamma assay were consecutively recruited. We compared the efficacy of the two tests in detecting latent TB infection and defined a more appropriate TST cutoff point. There was little agreement between the tests (k = 0.33, P < 0.0001): 53% of the contacts with a positive TST were ELISPOT negative, and 7% with a negative TST were ELISPOT positive. Furthermore, respectively 76 and 59% of the ELISPOT-negative contacts responded in vitro to BCG and PPD, suggesting that most of them were BCG vaccinated or infected with nontuberculous mycobacteria. The number of spot-forming cells significantly correlated with TST induration (P < 0.0001). Our in-house ELISPOT assay based on a restricted pool of highly selected peptides is more accurate than TST for identifying individuals with latent TB infection and could improve chemoprophylaxis for the control of TB.
Figures

Similar articles
-
[Evolution of IGRA researches].Kekkaku. 2008 Sep;83(9):641-52. Kekkaku. 2008. PMID: 18979999 Review. Japanese.
-
[Characteristics of a diagnostic method for tuberculosis infection based on whole blood interferon-gamma assay].Kekkaku. 2006 Nov;81(11):681-6. Kekkaku. 2006. PMID: 17154047 Review. Japanese.
-
[Comparison of interferon-gamma whole blood assay with tuberculin skin test for the diagnosis of tuberculosis infection in tuberculosis contacts].Mikrobiyol Bul. 2007 Apr;41(2):193-202. Mikrobiyol Bul. 2007. PMID: 17682705 Turkish.
-
Region of difference 1 antigen-specific CD4+ memory T cells correlate with a favorable outcome of tuberculosis.J Infect Dis. 2006 Oct 1;194(7):984-92. doi: 10.1086/507427. Epub 2006 Aug 30. J Infect Dis. 2006. PMID: 16960787
-
Interferon gamma release assays: principles and practice.Enferm Infecc Microbiol Clin. 2010 Apr;28(4):245-52. doi: 10.1016/j.eimc.2009.05.012. Epub 2009 Sep 24. Enferm Infecc Microbiol Clin. 2010. PMID: 19783328 Review.
Cited by
-
Comparison of an in-house and a commercial RD1-based ELISPOT-IFN-gamma assay for the diagnosis of Mycobacterium tuberculosis infection.Clin Med Res. 2006 Dec;4(4):266-72. doi: 10.3121/cmr.4.4.266. Clin Med Res. 2006. PMID: 17210976 Free PMC article. Clinical Trial.
-
Assessment of Mycobacterium tuberculosis OmpATb as a novel antigen for the diagnosis of bovine tuberculosis.Clin Vaccine Immunol. 2009 Sep;16(9):1314-21. doi: 10.1128/CVI.00151-09. Epub 2009 Jul 8. Clin Vaccine Immunol. 2009. PMID: 19587150 Free PMC article.
-
Screening for tuberculosis infection among newly arrived asylum seekers: comparison of QuantiFERONTB Gold with tuberculin skin test.BMC Infect Dis. 2008 May 14;8:65. doi: 10.1186/1471-2334-8-65. BMC Infect Dis. 2008. PMID: 18479508 Free PMC article.
-
ELISPOT-IFN-gamma assay instead of tuberculin skin test for detecting latent Mycobacterium tuberculosis infection in rheumatic patients candidate to anti-TNF-alpha treatment.Clin Rheumatol. 2010 Oct;29(10):1135-41. doi: 10.1007/s10067-010-1532-1. Epub 2010 Jul 20. Clin Rheumatol. 2010. PMID: 20645117
-
The performance of T-cell Xtend reagent in increasing blood storage times for interferon gamma release assays.J Clin Lab Anal. 2018 Feb;32(2):e22253. doi: 10.1002/jcla.22253. Epub 2017 Jul 3. J Clin Lab Anal. 2018. PMID: 28670691 Free PMC article.
References
-
- American Thoracic Society. 2000. Targeted tuberculin testing and treatment of latent tuberculosis infection. Joint statement of the American Thoracic Society and the Centers for Disease Control and Prevention, endorsed by the Council of the Infectious Diseases Society of America in September 1999. Am. J. Respir. Crit. Care Med. 161:S221-S247. - PubMed
-
- American Thoracic Society. 2000. Diagnostic standards and classification of tuberculosis in adults and children. Joint statement of the American Thoracic Society and the Centers for Disease Control and Prevention, endorsed by the Council of the Infectious Diseases Society of America in September 1999. Am. J. Respir. Crit. Care Med. 161:1376-1395. - PubMed
-
- Andersen, P., M. E. Munk, J. M. Pollock, and T. M. Doherty. 2000. Specific immune-based diagnosis of tuberculosis. Lancet 356:1099-1104. - PubMed
-
- Arend, S. M., P. Andersen, K. E. van Meijgaarden, R. L. Skjot, Y. W. Subronto, J. T. van Dissel, and T. H. Ottenhoff. 2000. Detection of active tuberculosis infection by T-cell responses to early-secreted antigenic target 6-kDa protein and culture filtrate protein 10. J. Infect. Dis. 181:1850-1854. - PubMed
-
- Arend, S. M., A. Geluk, K. E. van Meijgaarden, J. T. van Dissel, M. Theisen, P. Andersen, and T. H. Ottenhoff. 2000. Antigenic equivalence of human T-cell responses to Mycobacterium tuberculosis-specific RD1-encoded protein antigens ESAT-6 and culture filtrate protein 10 and to mixtures of synthetic peptides. Infect. Immun. 68:3314-3321. - PMC - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Miscellaneous

