Glucosylceramide synthase is an essential regulator of pathogenicity of Cryptococcus neoformans
- PMID: 16741577
- PMCID: PMC1466548
- DOI: 10.1172/JCI27890
Glucosylceramide synthase is an essential regulator of pathogenicity of Cryptococcus neoformans
Erratum in
- J Clin Invest. 2007 May;117(5):1450
Abstract
The pathogenic fungus Cryptococcus neoformans infects humans upon inhalation and causes the most common fungal meningoencephalitis in immunocompromised subjects worldwide. In the host, C. neoformans is found both intracellularly and extracellularly, but how these two components contribute to the development of the disease is largely unknown. Here we show that the glycosphingolipid glucosylceramide (GlcCer), which is present in C. neoformans, was essential for fungal growth in host extracellular environments, such as in alveolar spaces and in the bloodstream, which are characterized by a neutral/alkaline pH, but not in the host intracellular environment, such as in the phagolysosome of macrophages, which is characteristically acidic. Indeed, a C. neoformans mutant strain lacking GlcCer did not grow in vitro at a neutral/alkaline pH, yet it had no growth defect at an acidic pH. The mechanism by which GlcCer regulates alkali tolerance was by allowing the transition of C. neoformans through the cell cycle. This study establishes C. neoformans GlcCer as a key virulence factor of cryptococcal pathogenicity, with important implications for future development of new antifungal strategies.
Figures
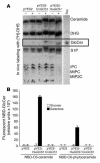



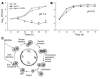
Comment in
-
Cryptococcal virulence: beyond the usual suspects.J Clin Invest. 2006 Jun;116(6):1481-3. doi: 10.1172/JCI28842. J Clin Invest. 2006. PMID: 16741574 Free PMC article.
Similar articles
-
Cryptococcal virulence: beyond the usual suspects.J Clin Invest. 2006 Jun;116(6):1481-3. doi: 10.1172/JCI28842. J Clin Invest. 2006. PMID: 16741574 Free PMC article.
-
The Transcription Factor Pdr802 Regulates Titan Cell Formation and Pathogenicity of Cryptococcus neoformans.mBio. 2021 Mar 9;12(2):e03457-20. doi: 10.1128/mBio.03457-20. mBio. 2021. PMID: 33688010 Free PMC article.
-
Methylation of glycosylated sphingolipid modulates membrane lipid topography and pathogenicity of Cryptococcus neoformans.Cell Microbiol. 2012 Apr;14(4):500-16. doi: 10.1111/j.1462-5822.2011.01735.x. Epub 2012 Jan 9. Cell Microbiol. 2012. PMID: 22151739 Free PMC article.
-
Induction of signal transduction pathways related to the pathogenicity of Cryptococcus neoformans in the host environment.Drug Discov Ther. 2019;13(4):177-182. doi: 10.5582/ddt.2019.01047. Drug Discov Ther. 2019. PMID: 31534068 Review.
-
The Environmental Effects on Virulence Factors and the Antifungal Susceptibility of Cryptococcus neoformans.Int J Mol Sci. 2021 Jun 11;22(12):6302. doi: 10.3390/ijms22126302. Int J Mol Sci. 2021. PMID: 34208294 Free PMC article. Review.
Cited by
-
Characterization of N-Acetylglucosamine Biosynthesis in Pneumocystis species. A New Potential Target for Therapy.Am J Respir Cell Mol Biol. 2017 Feb;56(2):213-222. doi: 10.1165/rcmb.2016-0155OC. Am J Respir Cell Mol Biol. 2017. PMID: 27632412 Free PMC article.
-
Proteins interacting with mitochondrial ATP-dependent Lon protease (MAP1) in Magnaporthe oryzae are involved in rice blast disease.Mol Plant Pathol. 2015 Oct;16(8):847-59. doi: 10.1111/mpp.12242. Epub 2015 Mar 21. Mol Plant Pathol. 2015. PMID: 25605006 Free PMC article.
-
Sphingolipid signaling in fungal pathogens.Adv Exp Med Biol. 2010;688:232-7. doi: 10.1007/978-1-4419-6741-1_16. Adv Exp Med Biol. 2010. PMID: 20919658 Free PMC article. Review.
-
The cryptococcal enzyme inositol phosphosphingolipid-phospholipase C confers resistance to the antifungal effects of macrophages and promotes fungal dissemination to the central nervous system.Infect Immun. 2006 Oct;74(10):5977-88. doi: 10.1128/IAI.00768-06. Infect Immun. 2006. PMID: 16988277 Free PMC article.
-
The endocytic adaptor proteins of pathogenic fungi: charting new and familiar pathways.Med Mycol. 2011 Jul;49(5):449-57. doi: 10.3109/13693786.2011.553246. Epub 2011 Jan 24. Med Mycol. 2011. PMID: 21254965 Free PMC article. Review.
References
-
- Casadevall A., Perfect J.R.1998. . Cryptococcus neoformans. ASM Press.Washington, DC, USA.351–380.
-
- Feldmesser M., Tucker S., Casadevall A. Intracellular parasitism of macrophages byCryptococcus neoformans . . Trends Microbiol. 2001;9:273–278. - PubMed
-
- Chretien F., et al. Pathogenesis of cerebralCryptococcus neoformans infection after fungemia. . J. Infect. Dis. 2002;186:522–530. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

