Increased hematopoietic stem cell mobilization in aged mice
- PMID: 16741255
- PMCID: PMC1895568
- DOI: 10.1182/blood-2005-12-010272
Increased hematopoietic stem cell mobilization in aged mice
Abstract
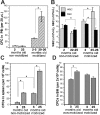
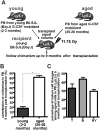
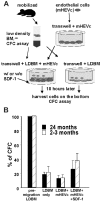
Hematopoietic stem and progenitor cells (HSPCs) are located in the bone marrow in close association with a highly organized 3-dimensional structure formed by stroma cells, referred to as the niche. Mobilization of HSPCs from bone marrow to peripheral blood in response to granulocyte colony-stimulating factor (G-CSF) requires de-adhesion of HSPCs from the niche. The influence of aging of HSPCs on cell-stroma interactions has not been determined in detail. Using a mouse model of G-CSF-induced mobilization, we demonstrated that the ability to mobilize hematopoietic stem cells is approximately 5-fold greater in aged mice. Competitive mobilization experiments confirmed that enhanced mobilization ability was intrinsic to the stem cell. Enhanced mobilization efficiency of primitive hematopoietic cells from aged mice correlated with reduced adhesion of hematopoietic progenitor cells to stroma and with elevated levels of GTP-bound Cdc42. These results might indicate that stroma-stem cell interactions are dynamic over a lifetime and result in physiologically relevant changes in the biology of primitive hematopoietic cells with age.
Figures

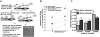
Similar articles
-
MicroRNA126 contributes to granulocyte colony-stimulating factor-induced hematopoietic progenitor cell mobilization by reducing the expression of vascular cell adhesion molecule 1.Haematologica. 2012 Jun;97(6):818-26. doi: 10.3324/haematol.2011.056945. Epub 2012 Jan 22. Haematologica. 2012. PMID: 22271895 Free PMC article.
-
Rational identification of a Cdc42 inhibitor presents a new regimen for long-term hematopoietic stem cell mobilization.Leukemia. 2019 Mar;33(3):749-761. doi: 10.1038/s41375-018-0251-5. Epub 2018 Sep 25. Leukemia. 2019. PMID: 30254339 Free PMC article.
-
Pharmacological inhibition of EGFR signaling enhances G-CSF-induced hematopoietic stem cell mobilization.Nat Med. 2010 Oct;16(10):1141-6. doi: 10.1038/nm.2217. Epub 2010 Sep 26. Nat Med. 2010. PMID: 20871610 Free PMC article.
-
Cytokine-induced hematopoietic stem and progenitor cell mobilization: unraveling interactions between stem cells and their niche.Ann N Y Acad Sci. 2020 Apr;1466(1):24-38. doi: 10.1111/nyas.14059. Epub 2019 Apr 21. Ann N Y Acad Sci. 2020. PMID: 31006885 Free PMC article. Review.
-
The role of granulocyte colony-stimulating factor in mobilization and transplantation of peripheral blood progenitor and stem cells.Cytokines Mol Ther. 1995 Dec;1(4):249-70. Cytokines Mol Ther. 1995. PMID: 9384679 Review.
Cited by
-
Concise review: hematopoietic stem cell aging, life span, and transplantation.Stem Cells Transl Med. 2012 Sep;1(9):651-7. doi: 10.5966/sctm.2012-0033. Epub 2012 Sep 5. Stem Cells Transl Med. 2012. PMID: 23197871 Free PMC article. Review.
-
Haematopoietic ageing through the lens of single-cell technologies.Dis Model Mech. 2021 Jan 22;14(1):dmm047340. doi: 10.1242/dmm.047340. Dis Model Mech. 2021. PMID: 33735102 Free PMC article. Review.
-
Altered cellular dynamics and endosteal location of aged early hematopoietic progenitor cells revealed by time-lapse intravital imaging in long bones.Blood. 2009 Jul 9;114(2):290-8. doi: 10.1182/blood-2008-12-195644. Epub 2009 Apr 8. Blood. 2009. PMID: 19357397 Free PMC article.
-
Comparison of endothelial cell phenotypic markers of late-outgrowth endothelial progenitor cells isolated from patients with coronary artery disease and healthy volunteers.Tissue Eng Part A. 2009 Nov;15(11):3473-86. doi: 10.1089/ten.TEA.2008.0673. Tissue Eng Part A. 2009. PMID: 19435420 Free PMC article.
-
Hematopoietic stem cell niche maintenance during homeostasis and regeneration.Nat Med. 2014 Aug;20(8):833-46. doi: 10.1038/nm.3647. Nat Med. 2014. PMID: 25100529 Free PMC article. Review.
References
-
- Zhang J, Niu C, Ye L, et al. Identification of the haematopoietic stem cell niche and control of the niche size. Nature. 2003;425: 836-841. - PubMed
-
- Calvi LM, Adams GB, Weibrecht KW, et al. Osteoblastic cells regulate the haematopoietic stem cell niche. Nature. 2003;425: 841-846. - PubMed
-
- Papayannopoulou T. Current mechanistic scenarios in hematopoietic stem/progenitor cell mobilization. Blood. 2004;103: 1580-1585. - PubMed
-
- Lapidot T, Petit I. Current understanding of stem cell mobilization: the roles of chemokines, proteolytic enzymes, adhesion molecules, cytokines, and stromal cells. Exp Hematol. 2002;30: 973-981. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

