Efficient inhibition of EGFR signaling and of tumour growth by antagonistic anti-EFGR Nanobodies
- PMID: 16738850
- PMCID: PMC11030579
- DOI: 10.1007/s00262-006-0180-4
Efficient inhibition of EGFR signaling and of tumour growth by antagonistic anti-EFGR Nanobodies
Abstract
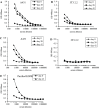
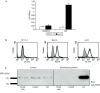
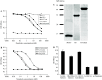
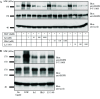
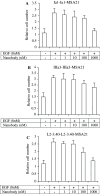
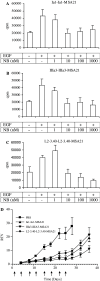
The development of a number of different solid tumours is associated with over-expression of ErbB1, or the epidermal growth factor receptor (EGFR), and this over-expression is often correlated with poor prognosis of patients. Therefore, this receptor tyrosine kinase is considered to be an attractive target for antibody-based therapy. Indeed, antibodies to the EGFR have already proven their value for the treatment of several solid tumours, especially in combination with chemotherapeutic treatment regimens. Variable domains of camelid heavy chain-only antibodies (called Nanobodies) have superior properties compared with classical antibodies in that they are small, very stable, easy to produce in large quantities and easy to re-format into multi-valent or multi-specific proteins. Furthermore, they can specifically be selected for a desired function by phage antibody display. In this report, we describe the successful selection and the characterisation of antagonistic anti-EGFR Nanobodies. By using a functional selection strategy, Nanobodies that specifically competed for EGF binding to the EGFR were isolated from "immune" phage Nanobody repertoires. The selected antibody fragments were found to efficiently inhibit EGF binding to the EGFR without acting as receptor agonists themselves. In addition, they blocked EGF-mediated signalling and EGF-induced cell proliferation. In an in vivo murine xenograft model, the Nanobodies were effective in delaying the outgrowth of A431-derived solid tumours. This is the first report describing the successful use of untagged Nanobodies for the in vivo treatment of solid tumours. The results show that functional phage antibody selection, coupled to the rational design of Nanobodies, permits the rapid development of novel anti-cancer antibody-based therapeutics.
Figures
Similar articles
-
A biparatopic anti-EGFR nanobody efficiently inhibits solid tumour growth.Int J Cancer. 2011 Oct 15;129(8):2013-24. doi: 10.1002/ijc.26145. Epub 2011 Aug 8. Int J Cancer. 2011. PMID: 21520037 Free PMC article.
-
Llama-derived single variable domains (nanobodies) directed against chemokine receptor CXCR7 reduce head and neck cancer cell growth in vivo.J Biol Chem. 2013 Oct 11;288(41):29562-72. doi: 10.1074/jbc.M113.498436. Epub 2013 Aug 26. J Biol Chem. 2013. PMID: 23979133 Free PMC article.
-
Efficient growth inhibition of EGFR over-expressing tumor cells by an anti-EGFR nanobody.Mol Biol Rep. 2013 Dec;40(12):6737-45. doi: 10.1007/s11033-013-2790-1. Epub 2013 Sep 20. Mol Biol Rep. 2013. PMID: 24052234
-
[99mTc]Epidermal growth factor receptor–specific nanobody.2008 Apr 22 [updated 2008 May 13]. In: Molecular Imaging and Contrast Agent Database (MICAD) [Internet]. Bethesda (MD): National Center for Biotechnology Information (US); 2004–2013. 2008 Apr 22 [updated 2008 May 13]. In: Molecular Imaging and Contrast Agent Database (MICAD) [Internet]. Bethesda (MD): National Center for Biotechnology Information (US); 2004–2013. PMID: 20641885 Free Books & Documents. Review.
-
IRDye 800CW-Anti-epidermal growth factor receptor nanobody 7D12.2012 Apr 6 [updated 2012 Jul 12]. In: Molecular Imaging and Contrast Agent Database (MICAD) [Internet]. Bethesda (MD): National Center for Biotechnology Information (US); 2004–2013. 2012 Apr 6 [updated 2012 Jul 12]. In: Molecular Imaging and Contrast Agent Database (MICAD) [Internet]. Bethesda (MD): National Center for Biotechnology Information (US); 2004–2013. PMID: 22787693 Free Books & Documents. Review.
Cited by
-
CYpHER: catalytic extracellular targeted protein degradation with high potency and durable effect.Nat Commun. 2024 Oct 9;15(1):8731. doi: 10.1038/s41467-024-52975-2. Nat Commun. 2024. PMID: 39384759 Free PMC article.
-
High affinity nanobodies against human epidermal growth factor receptor selected on cells by E. coli display.MAbs. 2016 Oct;8(7):1286-1301. doi: 10.1080/19420862.2016.1216742. Epub 2016 Jul 29. MAbs. 2016. PMID: 27472381 Free PMC article.
-
A bispecific nanobody approach to leverage the potent and widely applicable tumor cytolytic capacity of Vγ9Vδ2-T cells.Oncoimmunology. 2017 Oct 20;7(1):e1375641. doi: 10.1080/2162402X.2017.1375641. eCollection 2017. Oncoimmunology. 2017. PMID: 29296532 Free PMC article.
-
A biparatopic anti-EGFR nanobody efficiently inhibits solid tumour growth.Int J Cancer. 2011 Oct 15;129(8):2013-24. doi: 10.1002/ijc.26145. Epub 2011 Aug 8. Int J Cancer. 2011. PMID: 21520037 Free PMC article.
-
Construction of IgG-Fab2 bispecific antibody via intein-mediated protein trans-splicing reaction.Sci Rep. 2023 Sep 25;13(1):15961. doi: 10.1038/s41598-023-43110-0. Sci Rep. 2023. PMID: 37749185 Free PMC article.
References
-
- Bleeker WK, van Lammerts Bueren JJ, van Ojik HH, Gerritsen AF, Pluyter M, Houtkamp M, Halk E, Goldstein J, Schuurman J, van Dijk MA, van de Winkel JG, Parren PW. Dual mode of action of a human anti-epidermal growth factor receptor monoclonal antibody for cancer therapy. J Immunol. 2004;173:4699. - PubMed
-
- Bremer E, Samplonius DF, van Genne L, Dijkstra MH, Kroesen BJ, de Leij LF, Helfrich W. Simultaneous inhibition of EGFR signaling and enhanced activation of TRAIL-R-mediated apoptosis induction by an scFv:sTRAIL fusion protein with specificity for human EGFR. J Biol Chem. 2005;280(11):10025–10033. doi: 10.1074/jbc.M413673200. - DOI - PubMed
-
- Bruell D, Stocker M, Huhn M, Redding N, Kupper M, Schumacher P, Paetz A, Bruns CJ, Haisma HJ, Fischer R, Finnern R, Barth S. The recombinant anti-EGF receptor immunotoxin 425(scFv)-ETA’ suppresses growth of a highly metastatic pancreatic carcinoma cell line. Int J Oncol. 2003;23:1179. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

