The synthesis and high-level expression of a beta2-adrenergic receptor gene in a tetracycline-inducible stable mammalian cell line
- PMID: 16731977
- PMCID: PMC2265096
- DOI: 10.1110/ps.062080006
The synthesis and high-level expression of a beta2-adrenergic receptor gene in a tetracycline-inducible stable mammalian cell line
Abstract
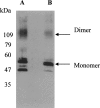
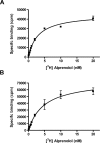
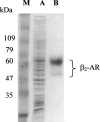
High-level expression of G-protein-coupled receptors (GPCRs) in functional form is required for structure-function studies. The main goal of the present work was to improve expression levels of beta2-adrenergic receptor (beta2-AR) so that biophysical studies involving EPR, NMR, and crystallography can be pursued. Toward this objective, the total synthesis of a codon-optimized hamster beta2-AR gene suitable for high-level expression in mammalian systems has been accomplished. Transient expression of the gene in COS-1 cells resulted in 18 +/- 3 pmol beta2-AR/mg of membrane protein, as measured by saturation binding assay using the beta2-AR antagonist [3H] dihydroalprenolol. Previously, we reported the development of an HEK293S tetracycline-inducible system for high-level expression of rhodopsin. Here, we describe construction of beta2-AR stable cell lines using the HEK293S-TetR-inducible system, which, after induction, express wild-type beta2-AR at levels of 220 +/- 40 pmol/mg of membrane protein corresponding to 50 +/- 8 microg/15-cm plate. This level of expression is the highest reported so far for any wild-type GPCR, other than rhodopsin. The yield of functional receptor using the single-step affinity purification is 12 +/- 3 microg/15-cm plate. This level of expression now makes it feasible to pursue structure-function studies using EPR. Furthermore, scale-up of beta2-AR expression using suspension cultures in a bioreactor should now allow production of enough beta2-AR for the application of biophysical techniques such as NMR spectroscopy and crystallography.
Figures

Similar articles
-
Cell-Free Expression, Purification, and Characterization of the Functional β2-Adrenergic Receptor for Multianalyte Detection of β-Agonists.Biochemistry (Mosc). 2017 Nov;82(11):1346-1353. doi: 10.1134/S0006297917110128. Biochemistry (Mosc). 2017. PMID: 29223161
-
High-level expression and purification of the human bradykinin B(2) receptor in a tetracycline-inducible stable HEK293S cell line.Protein Expr Purif. 2007 Oct;55(2):300-11. doi: 10.1016/j.pep.2007.04.020. Epub 2007 May 5. Protein Expr Purif. 2007. PMID: 17561413
-
Expression of glucagon receptors in tetracycline-inducible HEK293S GnT1- stable cell lines: an approach toward purification of receptor protein for structural studies.Biopolymers. 2008;90(3):287-96. doi: 10.1002/bip.20951. Biopolymers. 2008. PMID: 18260137
-
Expression systems for bovine rhodopsin: a review of the progress made in the Khorana laboratory.Biophys Rev. 2023 Jan 6;15(1):93-101. doi: 10.1007/s12551-022-01037-2. eCollection 2023 Feb. Biophys Rev. 2023. PMID: 36909956 Free PMC article. Review.
-
Bidirectional Role of β2-Adrenergic Receptor in Autoimmune Diseases.Front Pharmacol. 2018 Nov 27;9:1313. doi: 10.3389/fphar.2018.01313. eCollection 2018. Front Pharmacol. 2018. PMID: 30538630 Free PMC article. Review.
Cited by
-
Efficient expression screening of human membrane proteins in transiently transfected Human Embryonic Kidney 293S cells.Methods. 2011 Dec;55(4):273-80. doi: 10.1016/j.ymeth.2011.08.018. Epub 2011 Sep 8. Methods. 2011. PMID: 21925269 Free PMC article.
-
High-level expression in Saccharomyces cerevisiae enables isolation and spectroscopic characterization of functional human adenosine A2a receptor.J Struct Biol. 2007 Aug;159(2):166-78. doi: 10.1016/j.jsb.2007.05.001. Epub 2007 May 16. J Struct Biol. 2007. PMID: 17591446 Free PMC article.
-
Thromboxane receptor hyper-responsiveness in hypoxic pulmonary hypertension requires serine 324.Br J Pharmacol. 2014 Feb;171(3):676-87. doi: 10.1111/bph.12487. Br J Pharmacol. 2014. PMID: 24490858 Free PMC article.
-
Global fold of human cannabinoid type 2 receptor probed by solid-state 13C-, 15N-MAS NMR and molecular dynamics simulations.Proteins. 2014 Mar;82(3):452-65. doi: 10.1002/prot.24411. Epub 2013 Oct 17. Proteins. 2014. PMID: 23999926 Free PMC article.
-
Polyethyleneimine-based transient gene expression processes for suspension-adapted HEK-293E and CHO-DG44 cells.Protein Expr Purif. 2013 Nov;92(1):67-76. doi: 10.1016/j.pep.2013.09.001. Epub 2013 Sep 8. Protein Expr Purif. 2013. PMID: 24021764 Free PMC article.
References
-
- Babcock G.J., Mirzabekov T., Wojtowicz W., Sodroski J. 2001. Ligand binding characteristics of CXCR4 incorporated into paramagnetic proteoliposomes J. Biol. Chem. 276 38433–38440. - PubMed
-
- Benovic J.L., Shorr R.G., Caron M.G., Lefkowitz R.J. 1984. The mammalian β2-adrenergic receptor: Purification and characterization Biochemistry 23 4510–4518. - PubMed
-
- Bradel-Tretheway B.G., Zhen Z., Dewhurst S. 2003. Effects of codon-optimization on protein expression by the human herpesvirus 6 and 7 U51 open reading frame J. Virol. Methods 111 145–156. - PubMed
-
- Caron M.G., Srinivasan Y., Pitha J., Kociolek K., Lefkowitz R.J. 1979. Affinity chromatography of the β-adrenergic receptor J. Biol. Chem. 254 2923–2927. - PubMed
-
- Chelikani P., Kota P., Cao Z., Huang Y., Kim J., Reeves P.J., Khorana H.G. 2004. Expression, purification and crystallization trials on β2–adrenergic receptor FASEB J. 18–C281.
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

