Endosomal proteolysis by cathepsins is necessary for murine coronavirus mouse hepatitis virus type 2 spike-mediated entry
- PMID: 16731916
- PMCID: PMC1472567
- DOI: 10.1128/JVI.00442-06
Endosomal proteolysis by cathepsins is necessary for murine coronavirus mouse hepatitis virus type 2 spike-mediated entry
Abstract
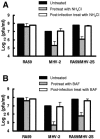
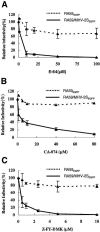
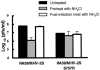
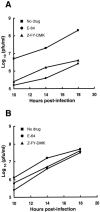
Most strains of murine coronavirus mouse hepatitis virus (MHV) express a cleavable spike glycoprotein that mediates viral entry and pH-independent cell-cell fusion. The MHV type 2 (MHV-2) strain of murine coronavirus differs from other strains in that it expresses an uncleaved spike and cannot induce cell-cell fusion at neutral pH values. We show here that while infection of the prototype MHV-A59 strain is not sensitive to pretreatment with lysosomotropic agents, MHV-2 replication is significantly inhibited by these agents. By use of an A59/MHV-2 chimeric virus, the susceptibility to lysosomotropic agents is mapped to the MHV-2 spike, suggesting a requirement of acidification of endosomes for MHV-2 spike-mediated entry. However, acidification is likely not a direct trigger for MHV-2 spike-mediated membrane fusion, as low-pH treatment is unable to overcome ammonium chloride inhibition, and it also cannot induce cell-cell fusion between MHV-2-infected cells. In contrast, trypsin treatment can both overcome ammonium chloride inhibition and promote cell-cell fusion. Inhibitors of the endosomal cysteine proteases cathepsin B and cathepsin L greatly reduce MHV-2 spike-mediated entry, while they have little effect on A59 entry, suggesting that there is a proteolytic step in MHV-2 entry. Finally, a recombinant virus expressing a cleaved MHV-2 spike has the ability to induce cell-cell fusion at neutral pH values and does not require low pH and endosomal cathepsins during infection. These studies demonstrate that endosomal proteolysis by cathepsins is necessary for MHV-2 spike-mediated entry; this is similar to the entry pathway recently described for severe acute respiratory syndrome coronavirus and indicates that coronaviruses may use multiple pathways for entry.
Figures




Similar articles
-
Role of endocytosis and low pH in murine hepatitis virus strain A59 cell entry.J Virol. 2007 Oct;81(19):10758-68. doi: 10.1128/JVI.00725-07. Epub 2007 Jul 11. J Virol. 2007. PMID: 17626088 Free PMC article.
-
Identification of H209 as Essential for pH 8-Triggered Receptor-Independent Syncytium Formation by S Protein of Mouse Hepatitis Virus A59.J Virol. 2018 May 14;92(11):e00209-18. doi: 10.1128/JVI.00209-18. Print 2018 Jun 1. J Virol. 2018. PMID: 29514915 Free PMC article.
-
Neurovirulent Murine Coronavirus JHM.SD Uses Cellular Zinc Metalloproteases for Virus Entry and Cell-Cell Fusion.J Virol. 2017 Mar 29;91(8):e01564-16. doi: 10.1128/JVI.01564-16. Print 2017 Apr 15. J Virol. 2017. PMID: 28148786 Free PMC article.
-
MHVR-independent cell-cell spread of mouse hepatitis virus infection requires neutral pH fusion.Adv Exp Med Biol. 1995;380:351-7. doi: 10.1007/978-1-4615-1899-0_57. Adv Exp Med Biol. 1995. PMID: 8830507 Review.
-
[Cell entry mechanisms of coronaviruses].Uirusu. 2009 Dec;59(2):215-22. doi: 10.2222/jsv.59.215. Uirusu. 2009. PMID: 20218330 Review. Japanese.
Cited by
-
Coronavirus Lung Infection Impairs Host Immunity against Secondary Bacterial Infection by Promoting Lysosomal Dysfunction.J Immunol. 2022 Oct 1;209(7):1314-1322. doi: 10.4049/jimmunol.2200198. Epub 2022 Sep 2. J Immunol. 2022. PMID: 36165196 Free PMC article.
-
Genetic comparison among various coronavirus strains for the identification of potential vaccine targets of SARS-CoV2.Infect Genet Evol. 2021 Apr;89:104490. doi: 10.1016/j.meegid.2020.104490. Epub 2020 Aug 1. Infect Genet Evol. 2021. PMID: 32745811 Free PMC article. Review.
-
3C protein of feline coronavirus inhibits viral replication independently of the autophagy pathway.Res Vet Sci. 2013 Dec;95(3):1241-7. doi: 10.1016/j.rvsc.2013.08.011. Epub 2013 Aug 30. Res Vet Sci. 2013. PMID: 24050534 Free PMC article.
-
Comparison of SARS-CoV-2 variants of concern in primary human nasal cultures demonstrates Delta as most cytopathic and Omicron as fastest replicating.bioRxiv [Preprint]. 2023 Dec 21:2023.08.24.553565. doi: 10.1101/2023.08.24.553565. bioRxiv. 2023. Update in: mBio. 2024 Apr 10;15(4):e0312923. doi: 10.1128/mbio.03129-23. PMID: 37662273 Free PMC article. Updated. Preprint.
-
Pathogenesis of neurotropic murine coronavirus is multifactorial.Trends Pharmacol Sci. 2011 Jan;32(1):2-7. doi: 10.1016/j.tips.2010.11.001. Epub 2010 Dec 7. Trends Pharmacol Sci. 2011. PMID: 21144598 Free PMC article.
References
-
- Chandran, K., and M. L. Nibert. 2003. Animal cell invasion by a large nonenveloped virus: reovirus delivers the goods. Trends Microbiol. 11:374-382. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

