HIV-1 Nef is associated with complex pulmonary vascular lesions in SHIV-nef-infected macaques
- PMID: 16728715
- PMCID: PMC2648120
- DOI: 10.1164/rccm.200601-005OC
HIV-1 Nef is associated with complex pulmonary vascular lesions in SHIV-nef-infected macaques
Abstract
Rationale: HIV-infected patients with pulmonary arterial hypertension have histologic manifestations that are indistinguishable from those found in patients with idiopathic pulmonary arterial hypertension. In addition, the role of pleiotropic viral proteins in the development of plexiform lesions in HIV-related pulmonary hypertension (HRPH) has not been explored. Simian immunodeficiency virus (SIV) infection of macaques has been found to closely recapitulate many of the characteristic features of HIV infection, and thus hallmarks of pulmonary arterial hypertension should also be found in this nonhuman primate model of HIV.
Objectives: To determine whether pulmonary arterial lesions were present in archived SIV-infected macaque lung tissues from Johns Hopkins University and two National Primate Research Centers.
Methods: Archived macaque and human lung sections were examined via immunohistochemistry for evidence of complex vascular lesions.
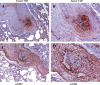
Results: Complex plexiform-like lesions characterized by lumenal obliteration, intimal disruption, medial hypertrophy, thrombosis, and recanalized lumena were found exclusively in animals infected with SHIV-nef (a chimeric viral construct containing the HIV nef gene in an SIV backbone), but not in animals infected with SIV. The mass of cells in the lesions were factor VIII positive, and contained cells positive for muscle-specific and smooth muscle actins. Lung mononuclear cells were positive for HIV Nef, suggesting viral replication. Endothelial cells in both the SHIV-nef macaques and patients with HRPH, but not in patients with idiopathic pulmonary arterial hypertension, were also Nef positive.
Conclusions: The discovery of complex vascular lesions in SHIV-nef- but not SIV-infected animals, and the presence of Nef in the vascular cells of patients with HRPH, suggest that Nef plays a key role in the development of severe pulmonary arterial disease.
Figures




Similar articles
-
Golgi dysfunction is a common feature in idiopathic human pulmonary hypertension and vascular lesions in SHIV-nef-infected macaques.Am J Physiol Lung Cell Mol Physiol. 2009 Oct;297(4):L729-37. doi: 10.1152/ajplung.00087.2009. Epub 2009 Jul 31. Am J Physiol Lung Cell Mol Physiol. 2009. PMID: 19648286 Free PMC article.
-
SIV/HIV Nef recombinant virus (SHIVnef) produces simian AIDS in rhesus macaques.Virology. 1999 Dec 20;265(2):235-51. doi: 10.1006/viro.1999.0051. Virology. 1999. PMID: 10600596
-
Chronology of genetic changes in the vpu, env, and Nef genes of chimeric simian-human immunodeficiency virus (strain HXB2) during acquisition of virulence for pig-tailed macaques.Virology. 1998 Sep 1;248(2):275-83. doi: 10.1006/viro.1998.9300. Virology. 1998. PMID: 9721236
-
From viral infection to pulmonary arterial hypertension: a role for viral proteins?AIDS. 2008 Sep;22 Suppl 3:S49-53. doi: 10.1097/01.aids.0000327516.55041.01. AIDS. 2008. PMID: 18845922 Review.
-
Could Nef and Vpr proteins contribute to disease progression by promoting depletion of bystander cells and prolonged survival of HIV-infected cells?Biochem Biophys Res Commun. 2000 Jan 27;267(3):677-85. doi: 10.1006/bbrc.1999.1708. Biochem Biophys Res Commun. 2000. PMID: 10673351 Review.
Cited by
-
HIV X4 Variants Increase Arachidonate 5-Lipoxygenase in the Pulmonary Microenvironment and are associated with Pulmonary Arterial Hypertension.Sci Rep. 2020 Jul 16;10(1):11696. doi: 10.1038/s41598-020-68060-9. Sci Rep. 2020. PMID: 32678115 Free PMC article.
-
Effect of cocaine on human immunodeficiency virus-mediated pulmonary endothelial and smooth muscle dysfunction.Am J Respir Cell Mol Biol. 2011 Jul;45(1):40-52. doi: 10.1165/rcmb.2010-0097OC. Epub 2010 Aug 27. Am J Respir Cell Mol Biol. 2011. PMID: 20802087 Free PMC article.
-
Infusion of HIV-1 Nef-expressing astrocytes into the rat hippocampus induces enteropathy and interstitial pneumonitis and increases blood-brain-barrier permeability.PLoS One. 2019 Nov 27;14(11):e0225760. doi: 10.1371/journal.pone.0225760. eCollection 2019. PLoS One. 2019. PMID: 31774879 Free PMC article.
-
An official ATS workshop report: Emerging issues and current controversies in HIV-associated pulmonary diseases.Proc Am Thorac Soc. 2011 Mar;8(1):17-26. doi: 10.1513/pats.2009-047WS. Proc Am Thorac Soc. 2011. PMID: 21364216 Free PMC article. Review.
-
Repression of Nrf2/ARE regulated antioxidant genes and dysregulation of the cellular redox environment by the HIV Transactivator of Transcription.Free Radic Biol Med. 2019 Sep;141:244-252. doi: 10.1016/j.freeradbiomed.2019.06.015. Epub 2019 Jun 22. Free Radic Biol Med. 2019. PMID: 31238128 Free PMC article.
References
-
- Kim KK, Factor SM. Membranoproliferative glomerulonephritis and plexogenic pulmonary arteriopathy in a homosexual man with acquired immunodeficiency syndrome. Hum Pathol 1987;18:1293–1296. - PubMed
-
- Speich R, Jenni R, Opravil M, Pfab M, Russi EW. Primary pulmonary hypertension in HIV infection. Chest 1991;100:1268–1271. - PubMed
-
- Mette SA, Palevsky HI, Pietra GG, Williams TM, Bruder E, Prestipino AJ, Patrick AM, Wirth JA. Primary pulmonary hypertension in association with human immunodeficiency virus infection: a possible viral etiology for some forms of hypertensive pulmonary arteriopathy. Am Rev Respir Dis 1992;145:1196–1200. - PubMed
-
- Pellicelli AM, Palmieri F, D'Ambrosio C, Rianda A, Boumis E, Girardi E, Antonucci G, D'Amato C, Borgia MC. Role of human immunodeficiency virus in primary pulmonary hypertension: case reports. Angiology 1998;49:1005–1011. - PubMed
-
- Sitbon O, Gressin V, Speich R, Macdonald PS, Opravil M, Cooper DA, Fourme T, Humbert M, Delfraissy JF, Simonneau G. Bosentan for the treatment of human immunodeficiency virus–associated pulmonary arterial hypertension. Am J Respir Crit Care Med 2004;170:1212–1217. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

