Nephritogenic lupus antibodies recognize glomerular basement membrane-associated chromatin fragments released from apoptotic intraglomerular cells
- PMID: 16723695
- PMCID: PMC1606630
- DOI: 10.2353/ajpath.2006.051329
Nephritogenic lupus antibodies recognize glomerular basement membrane-associated chromatin fragments released from apoptotic intraglomerular cells
Abstract
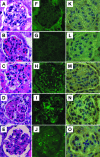
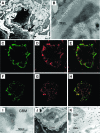
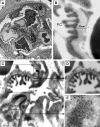
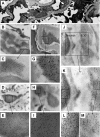
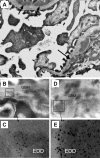
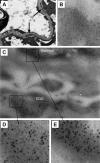
Antibodies to dsDNA represent a classification criterion for systemic lupus erythematosus. Subpopulations of these antibodies are involved in lupus nephritis. No known marker separates nephritogenic from non-nephritogenic anti-dsDNA antibodies. It is not clear whether specificity for glomerular target antigens or intrinsic antibody-affinity for dsDNA or nucleosomes is a critical parameter. Furthermore, it is still controversial whether glomerular target antigen(s) is constituted by nucleosomes or by non-nucleosomal glomerular structures. Previously, we have demonstrated that antibodies eluted from murine nephritic kidneys recognize nucleosomes, but not other glomerular antigens. In this study, we determined the structures that bind nephritogenic autoantibodies in vivo by transmission electron microscopy, immune electron microscopy, and colocalization immune electron microscopy using experimental antibodies to dsDNA, to histones and transcription factors, or to laminin. The data obtained are consistent and point at glomerular basement membrane-associated nucleosomes as target structures for the nephritogenic autoantibodies. Terminal deoxynucleotidyl-transferase-mediated dUTP nick end-labeling or caspase-3 assays demonstrate that lupus nephritis is linked to intraglomerular cell apoptosis. The data suggest that nucleosomes are released by apoptosis and associate with glomerulus basement membranes, which may then be targeted by pathogenic anti-nucleosome antibodies. Thus, apoptotic nucleosomes may represent both inducer and target structures for nephritogenic autoantibodies in systemic lupus erythematosus.
Figures

Similar articles
-
Critical comparative analyses of anti-alpha-actinin and glomerulus-bound antibodies in human and murine lupus nephritis.Arthritis Rheum. 2006 Mar;54(3):914-26. doi: 10.1002/art.21622. Arthritis Rheum. 2006. PMID: 16508974
-
Nucleosomes possess a high affinity for glomerular laminin and collagen IV and bind nephritogenic antibodies in murine lupus-like nephritis.Ann Rheum Dis. 2007 Dec;66(12):1661-8. doi: 10.1136/ard.2007.070482. Epub 2007 May 15. Ann Rheum Dis. 2007. PMID: 17504842 Free PMC article.
-
Nephritogenic antibodies bind in glomeruli through interaction with exposed chromatin fragments and not with renal cross-reactive antigens.Autoimmunity. 2011 Aug;44(5):373-83. doi: 10.3109/08916934.2010.541170. Epub 2011 Jan 19. Autoimmunity. 2011. PMID: 21244336
-
Nephritogenic potential of anti-DNA antibodies against necrotic nucleosomes.J Am Soc Nephrol. 2009 Apr;20(4):696-704. doi: 10.1681/ASN.2008010112. J Am Soc Nephrol. 2009. PMID: 19329762 Review.
-
A central role of nucleosomes in lupus nephritis.Ann N Y Acad Sci. 2007 Jun;1108:104-13. doi: 10.1196/annals.1422.012. Ann N Y Acad Sci. 2007. PMID: 17893976 Review.
Cited by
-
Anti-DNA autoantibodies initiate experimental lupus nephritis by binding directly to the glomerular basement membrane in mice.Kidney Int. 2012 Jul;82(2):184-92. doi: 10.1038/ki.2011.484. Epub 2012 Feb 1. Kidney Int. 2012. PMID: 22297676 Free PMC article.
-
Mechanisms of tissue injury in lupus nephritis.Arthritis Res Ther. 2011;13(6):250. doi: 10.1186/ar3528. Epub 2011 Dec 21. Arthritis Res Ther. 2011. PMID: 22192660 Free PMC article. Review.
-
Anti-dsDNA antibodies promote initiation, and acquired loss of renal Dnase1 promotes progression of lupus nephritis in autoimmune (NZBxNZW)F1 mice.PLoS One. 2009 Dec 29;4(12):e8474. doi: 10.1371/journal.pone.0008474. PLoS One. 2009. PMID: 20041189 Free PMC article.
-
Lupus nephritis: the central role of nucleosomes revealed.Am J Pathol. 2008 Feb;172(2):275-83. doi: 10.2353/ajpath.2008.070563. Epub 2008 Jan 10. Am J Pathol. 2008. PMID: 18187568 Free PMC article. Review.
-
Lupus nephritis progression in FcγRIIB-/-yaa mice is associated with early development of glomerular electron dense deposits and loss of renal DNase I in severe disease.PLoS One. 2017 Nov 30;12(11):e0188863. doi: 10.1371/journal.pone.0188863. eCollection 2017. PLoS One. 2017. PMID: 29190833 Free PMC article.
References
-
- Tan EM, Cohen AS, Fries JF, Masi AT, McShane DJ, Rothfield NF, Schaller JG, Talal N, Winchester RJ. The 1982 revised criteria for the classification of systemic lupus erythematosus. Arthritis Rheum. 1982;25:1271–1277. - PubMed
-
- Rekvig OP, Nossent JC. Anti-double-stranded DNA antibodies, nucleosomes, and systemic lupus erythematosus: a time for new paradigms? Arthritis Rheum. 2003;48:300–312. - PubMed
-
- Rekvig OP, Kalaaji M, Nossent H. Anti-DNA antibody subpopulations and lupus nephritis. Autoimmun Rev. 2004;3:1–6. - PubMed
-
- Waldman M, Madaio MP. Pathogenic autoantibodies in lupus nephritis. Lupus. 2005;14:19–24. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Research Materials

