Phosphorylation of JAK2 at serine 523: a negative regulator of JAK2 that is stimulated by growth hormone and epidermal growth factor
- PMID: 16705159
- PMCID: PMC1489095
- DOI: 10.1128/MCB.01591-05
Phosphorylation of JAK2 at serine 523: a negative regulator of JAK2 that is stimulated by growth hormone and epidermal growth factor
Abstract
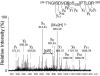
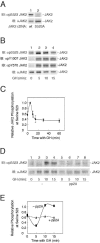
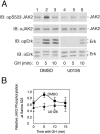
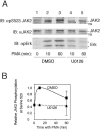
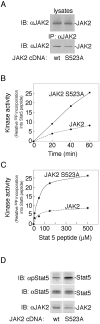
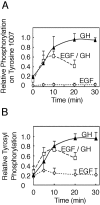
The tyrosine kinase JAK2 is a key signaling protein for at least 20 receptors in the cytokine/hematopoietin receptor superfamily and is a component of signaling for multiple receptor tyrosine kinases and several G-protein-coupled receptors. In this study, phosphopeptide affinity enrichment and mass spectrometry identified serine 523 (Ser523) in JAK2 as a site of phosphorylation. A phosphoserine 523 antibody revealed that Ser523 is rapidly but transiently phosphorylated in response to growth hormone (GH). MEK1 inhibitor UO126 suppresses GH-dependent phosphorylation of Ser523, suggesting that extracellular signal-regulated kinases (ERKs) 1 and/or 2 or another kinase downstream of MEK1 phosphorylate Ser523 in response to GH. Other ERK activators, phorbol 12-myristate 13-acetate and epidermal growth factor, also stimulate phosphorylation of Ser523. When Ser523 in JAK2 was mutated, JAK2 kinase activity as well as GH-dependent tyrosyl phosphorylation of JAK2 and Stat5 was enhanced, suggesting that phosphorylation of Ser523 inhibits JAK2 kinase activity. We hypothesize that phosphorylation of Ser523 in JAK2 by ERKs 1 and/or 2 or other as-yet-unidentified kinases acts in a negative feedback manner to dampen activation of JAK2 in response to GH and provides a mechanism by which prior exposure to environmental factors that regulate Ser523 phosphorylation might modulate the cell's response to GH.
Figures
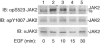

Similar articles
-
Phosphorylation of Jak2 on Ser(523) inhibits Jak2-dependent leptin receptor signaling.Mol Cell Biol. 2006 Jun;26(11):4063-73. doi: 10.1128/MCB.01589-05. Mol Cell Biol. 2006. PMID: 16705160 Free PMC article.
-
Growth hormone-induced phosphorylation of epidermal growth factor (EGF) receptor in 3T3-F442A cells. Modulation of EGF-induced trafficking and signaling.J Biol Chem. 2003 May 23;278(21):18902-13. doi: 10.1074/jbc.M300939200. Epub 2003 Mar 14. J Biol Chem. 2003. PMID: 12642595
-
Growth hormone-induced tyrosyl phosphorylation and deoxyribonucleic acid binding activity of Stat5A and Stat5B.Endocrinology. 1997 Aug;138(8):3426-34. doi: 10.1210/endo.138.8.5332. Endocrinology. 1997. PMID: 9231797
-
Growth hormone-induced tyrosine phosphorylation of EGF receptor as an essential element leading to MAP kinase activation and gene expression.Endocr J. 1998 Apr;45 Suppl:S27-31. doi: 10.1507/endocrj.45.suppl_s27. Endocr J. 1998. PMID: 9790226 Review.
-
SH2-B and SIRP: JAK2 binding proteins that modulate the actions of growth hormone.Recent Prog Horm Res. 2000;55:293-311. Recent Prog Horm Res. 2000. PMID: 11036942 Review.
Cited by
-
Phosphorylation of Jak2 on Ser(523) inhibits Jak2-dependent leptin receptor signaling.Mol Cell Biol. 2006 Jun;26(11):4063-73. doi: 10.1128/MCB.01589-05. Mol Cell Biol. 2006. PMID: 16705160 Free PMC article.
-
JAK redux: a second look at the regulation and role of JAKs in the heart.Am J Physiol Heart Circ Physiol. 2009 Nov;297(5):H1545-56. doi: 10.1152/ajpheart.00032.2009. Epub 2009 Aug 28. Am J Physiol Heart Circ Physiol. 2009. PMID: 19717737 Free PMC article. Review.
-
Regulation of Jak2 function by phosphorylation of Tyr317 and Tyr637 during cytokine signaling.Mol Cell Biol. 2009 Jun;29(12):3367-78. doi: 10.1128/MCB.00278-09. Epub 2009 Apr 13. Mol Cell Biol. 2009. PMID: 19364823 Free PMC article.
-
Janus kinase 3: the controller and the controlled.Acta Biochim Biophys Sin (Shanghai). 2012 Mar;44(3):187-96. doi: 10.1093/abbs/gmr105. Epub 2011 Nov 29. Acta Biochim Biophys Sin (Shanghai). 2012. PMID: 22130498 Free PMC article. Review.
-
Interleukin-2 Receptor β Thr-450 Phosphorylation Is a Positive Regulator for Receptor Complex Stability and Activation of Signaling Molecules.J Biol Chem. 2015 Aug 21;290(34):20972-20983. doi: 10.1074/jbc.M115.660654. Epub 2015 Jul 7. J Biol Chem. 2015. PMID: 26152718 Free PMC article.
References
-
- Argetsinger, L. S., N. Billestrup, G. Norstedt, M. F. White, and C. Carter-Su. 1996. Growth hormone, interferon-gamma, and leukemia inhibitory factor utilize insulin receptor substrate-2 in intracellular signaling. J. Biol. Chem. 271:29415-29421. - PubMed
-
- Argetsinger, L. S., G. S. Campbell, X. Yang, B. A. Witthuhn, O. Silvennoinen, J. N. Ihle, and C. Carter-Su. 1993. Identification of JAK2 as a growth hormone receptor-associated tyrosine kinase. Cell 74:237-244. - PubMed
-
- Argetsinger, L. S., and C. Carter-Su. 1996. Mechanism of signaling by growth hormone receptor. Physiol. Rev. 76:1089-1107. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous
