Nuclear trafficking of Drosophila Frizzled-2 during synapse development requires the PDZ protein dGRIP
- PMID: 16682643
- PMCID: PMC1472532
- DOI: 10.1073/pnas.0600387103
Nuclear trafficking of Drosophila Frizzled-2 during synapse development requires the PDZ protein dGRIP
Abstract
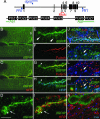
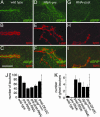
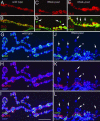
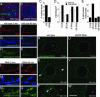
The Wingless pathway plays an essential role during synapse development. Recent studies at Drosophila glutamatergic synapses suggest that Wingless is secreted by motor neuron terminals and binds to postsynaptic Drosophila Frizzled-2 (DFz2) receptors. DFz2 is, in turn, endocytosed and transported to the muscle perinuclear area, where it is cleaved, and the C-terminal fragment is imported into the nucleus, presumably to regulate transcription during synapse growth. Alterations in this pathway interfere with the formation of new synaptic boutons and lead to aberrant synaptic structures. Here, we show that the 7 PDZ protein dGRIP is necessary for the trafficking of DFz2 to the nucleus. dGRIP is localized to Golgi and trafficking vesicles, and dgrip mutants mimic the synaptic phenotypes observed in wg and dfz2 mutants. DFz2 and dGRIP colocalize in trafficking vesicles, and a severe decrease in dGRIP levels prevents the transport of endocytosed DFz2 receptors to the nucleus. Moreover, coimmunoprecipitation experiments in transfected cells and yeast two-hybrid assays suggest that the C terminus of DFz2 interacts directly with the PDZ domains 4 and 5. These results provide a mechanism by which DFz2 is transported from the postsynaptic membrane to the postsynaptic nucleus during synapse formation and implicate dGRIP as an essential molecule in the transport of this signal.
Conflict of interest statement
Conflict of interest statement: No conflicts declared.
Figures

Similar articles
-
Trans-synaptic transmission of vesicular Wnt signals through Evi/Wntless.Cell. 2009 Oct 16;139(2):393-404. doi: 10.1016/j.cell.2009.07.051. Cell. 2009. PMID: 19837038 Free PMC article.
-
Wingless signaling at synapses is through cleavage and nuclear import of receptor DFrizzled2.Science. 2005 Nov 25;310(5752):1344-7. doi: 10.1126/science.1117051. Science. 2005. PMID: 16311339 Free PMC article.
-
The nuclear import of Frizzled2-C by Importins-beta11 and alpha2 promotes postsynaptic development.Nat Neurosci. 2010 Aug;13(8):935-43. doi: 10.1038/nn.2593. Epub 2010 Jul 4. Nat Neurosci. 2010. PMID: 20601947 Free PMC article.
-
Cell adhesion molecules in Drosophila synapse development and function.Sci China Life Sci. 2012 Jan;55(1):20-6. doi: 10.1007/s11427-012-4273-3. Epub 2012 Feb 8. Sci China Life Sci. 2012. PMID: 22314487 Review.
-
Wingless signaling: the inconvenient complexities of life.Curr Biol. 1998 Feb 12;8(4):R140-4. doi: 10.1016/s0960-9822(98)70081-8. Curr Biol. 1998. PMID: 9501979 Review. No abstract available.
Cited by
-
Presynaptic establishment of the synaptic cleft extracellular matrix is required for post-synaptic differentiation.Genes Dev. 2007 Oct 15;21(20):2607-28. doi: 10.1101/gad.1574107. Epub 2007 Sep 27. Genes Dev. 2007. PMID: 17901219 Free PMC article.
-
The glycosphingolipid MacCer promotes synaptic bouton formation in Drosophila by interacting with Wnt.Elife. 2018 Oct 25;7:e38183. doi: 10.7554/eLife.38183. Elife. 2018. PMID: 30355446 Free PMC article.
-
Carrier of Wingless (Cow) Regulation of Drosophila Neuromuscular Junction Development.eNeuro. 2020 Mar 10;7(2):ENEURO.0285-19.2020. doi: 10.1523/ENEURO.0285-19.2020. Print 2020 Mar/Apr. eNeuro. 2020. PMID: 32024666 Free PMC article.
-
Mutations in Wnt2 alter presynaptic motor neuron morphology and presynaptic protein localization at the Drosophila neuromuscular junction.PLoS One. 2010 Sep 15;5(9):e12778. doi: 10.1371/journal.pone.0012778. PLoS One. 2010. PMID: 20856675 Free PMC article.
-
Born to run: creating the muscle fiber.Curr Opin Cell Biol. 2010 Oct;22(5):566-74. doi: 10.1016/j.ceb.2010.08.009. Curr Opin Cell Biol. 2010. PMID: 20817426 Free PMC article. Review.
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

