Laser-assisted blastocyst dissection and subsequent cultivation of embryonic stem cells in a serum/cell free culture system: applications and preliminary results in a murine model
- PMID: 16681851
- PMCID: PMC1479373
- DOI: 10.1186/1479-5876-4-20
Laser-assisted blastocyst dissection and subsequent cultivation of embryonic stem cells in a serum/cell free culture system: applications and preliminary results in a murine model
Abstract
Background: To evaluate embryonic stem cell (ESC) harvesting methods with an emphasis on derivation of ESC lines without feeder cells or sera. Using a murine model, laser-assisted blastocyst dissection was performed and compared to conventional immunosurgery to assess a novel laser application for inner cell mass (ICM) isolation.
Methods: Intact blastocysts or isolated ICMs generated in a standard mouse strain were plated in medium with or without serum to compare ESC harvesting efficiency. ESC derivation was also undertaken in a feeder cell-free culture system.
Results: Although ICM growth and dissociation was comparable irrespective of the media components, an enhanced ESC harvest was observed in our serum-free medium (p < 0.01). ESC harvest rate was not affected by ICM isolation technique but was attenuated in the feeder cell-free group.
Conclusion: Achieving successful techniques for human ESC research is fundamentally dependent on preliminary work using experimental animals. In this study, all experimentally developed ESC lines manifested similar features to ESCs obtained from intact blastocysts in standard culture. Cell/sera free murine ESC harvest and propagation are feasible procedures for an embryology laboratory and await refinements for translation to human medical research.
Figures
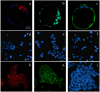





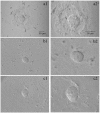
Similar articles
-
Selection of appropriate isolation method based on morphology of blastocyst for efficient derivation of buffalo embryonic stem cells.Cytotechnology. 2014 Mar;66(2):239-50. doi: 10.1007/s10616-013-9561-7. Epub 2013 Apr 4. Cytotechnology. 2014. PMID: 23553019 Free PMC article.
-
Whole-blastocyst culture followed by laser drilling technology enhances the efficiency of inner cell mass isolation and embryonic stem cell derivation from good- and poor-quality mouse embryos: new insights for derivation of human embryonic stem cell lines.Stem Cells Dev. 2008 Apr;17(2):255-67. doi: 10.1089/scd.2007.0157. Stem Cells Dev. 2008. PMID: 18447641
-
Derivation and characterization of novel nonhuman primate embryonic stem cell lines from in vitro-fertilized baboon preimplantation embryos.Stem Cells Dev. 2011 Jun;20(6):1053-62. doi: 10.1089/scd.2010.0372. Epub 2010 Nov 4. Stem Cells Dev. 2011. PMID: 20874104
-
[Human embryonic stem cells. Problems and perspectives].Tsitologiia. 2007;49(7):529-37. Tsitologiia. 2007. PMID: 17918336 Review. Russian.
-
Searching for naïve human pluripotent stem cells.World J Stem Cells. 2015 Apr 26;7(3):649-56. doi: 10.4252/wjsc.v7.i3.649. World J Stem Cells. 2015. PMID: 25914771 Free PMC article. Review.
Cited by
-
Laser-assisted Lentiviral Gene Delivery to Mouse Fertilized Eggs.J Vis Exp. 2018 Nov 1;(141):10.3791/58327. doi: 10.3791/58327. J Vis Exp. 2018. PMID: 30451224 Free PMC article.
-
Promoter analysis of the rabbit POU5F1 gene and its expression in preimplantation stage embryos.BMC Mol Biol. 2009 Sep 4;10:88. doi: 10.1186/1471-2199-10-88. BMC Mol Biol. 2009. PMID: 19732419 Free PMC article.
-
Endothelial differentiation of embryonic stem cells.Curr Protoc Stem Cell Biol. 2008 Sep;Chapter 1:Unit 1F.5. doi: 10.1002/9780470151808.sc01f05s6. Curr Protoc Stem Cell Biol. 2008. PMID: 18819086 Free PMC article.
-
Shrinky-dink hanging drops: a simple way to form and culture embryoid bodies.J Vis Exp. 2008 Mar 5;(13):692. doi: 10.3791/692. J Vis Exp. 2008. PMID: 19066572 Free PMC article.
-
Role of the embryology laboratory in the human embryonic stem cell line derivation process.Cytotechnology. 2006 Sep;52(1):1-11. doi: 10.1007/s10616-006-9031-6. Epub 2006 Nov 25. Cytotechnology. 2006. PMID: 19002861 Free PMC article.
References
-
- Robertson EJ. Practical approach series. IRL Press Oxford, UK; 1987. Teratocarcinomas and embryonic stem cells: a practical approach.
LinkOut - more resources
Full Text Sources
Other Literature Sources

