ATM-Chk2-p53 activation prevents tumorigenesis at an expense of organ homeostasis upon Brca1 deficiency
- PMID: 16675955
- PMCID: PMC1462967
- DOI: 10.1038/sj.emboj.7601115
ATM-Chk2-p53 activation prevents tumorigenesis at an expense of organ homeostasis upon Brca1 deficiency
Abstract
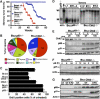
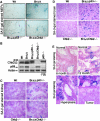
BRCA1 is a checkpoint and DNA damage repair gene that secures genome integrity. We have previously shown that mice lacking full-length Brca1 (Brca1(delta11/delta11)) die during embryonic development. Haploid loss of p53 completely rescues embryonic lethality, and adult Brca1(delta11/delta11)p53+/- mice display cancer susceptibility and premature aging. Here, we show that reduced expression and/or the absence of Chk2 allow Brca1(delta11/delta11) mice to escape from embryonic lethality. Compared to Brca1(delta11/delta11)p53+/- mice, lifespan of Brca1(delta11/delta11)Chk2-/- mice was remarkably extended. Analysis of Brca1(delta11/delta11)Chk2-/- mice revealed that p53-dependent apoptosis and growth defect caused by Brca1 deficiency are significantly attenuated in rapidly proliferating organs. However, in later life, Brca1(delta11/delta11)Chk2-/- female mice developed multiple tumors. Furthermore, haploid loss of ATM also rescued Brca1 deficiency-associated embryonic lethality and premature aging. Thus, in response to Brca1 deficiency, the activation of the ATM-Chk2-p53 signaling pathway contributes to the suppression of neoplastic transformation, while leading to compromised organismal homeostasis. Our data highlight how accurate maintenance of genomic integrity is critical for the suppression of both aging and malignancy, and provide a further link between aging and cancer.
Figures

Similar articles
-
A selective requirement for 53BP1 in the biological response to genomic instability induced by Brca1 deficiency.Mol Cell. 2009 Aug 28;35(4):534-41. doi: 10.1016/j.molcel.2009.06.037. Mol Cell. 2009. PMID: 19716796 Free PMC article.
-
ATR-Chk2 signaling in p53 activation and DNA damage response during cisplatin-induced apoptosis.J Biol Chem. 2008 Mar 7;283(10):6572-83. doi: 10.1074/jbc.M707568200. Epub 2007 Dec 27. J Biol Chem. 2008. PMID: 18162465
-
STAT-1 facilitates the ATM activated checkpoint pathway following DNA damage.J Cell Sci. 2005 Apr 15;118(Pt 8):1629-39. doi: 10.1242/jcs.01728. Epub 2005 Mar 22. J Cell Sci. 2005. Retraction in: J Cell Sci. 2015 Mar 1;128(5):1064. doi: 10.1242/jcs.169243. PMID: 15784679 Retracted.
-
The ATM-Chk2 and ATR-Chk1 pathways in DNA damage signaling and cancer.Adv Cancer Res. 2010;108:73-112. doi: 10.1016/B978-0-12-380888-2.00003-0. Adv Cancer Res. 2010. PMID: 21034966 Review.
-
p53: guardian of the genome and policeman of the oncogenes.Cell Cycle. 2007 May 2;6(9):1006-10. doi: 10.4161/cc.6.9.4211. Epub 2007 May 28. Cell Cycle. 2007. PMID: 17457049 Review.
Cited by
-
Trial Watch: Targeting ATM-CHK2 and ATR-CHK1 pathways for anticancer therapy.Mol Cell Oncol. 2015 Feb 23;2(4):e1012976. doi: 10.1080/23723556.2015.1012976. eCollection 2015 Oct-Dec. Mol Cell Oncol. 2015. PMID: 27308506 Free PMC article. Review.
-
53BP1 inhibits homologous recombination in Brca1-deficient cells by blocking resection of DNA breaks.Cell. 2010 Apr 16;141(2):243-54. doi: 10.1016/j.cell.2010.03.012. Epub 2010 Apr 1. Cell. 2010. PMID: 20362325 Free PMC article.
-
Impaired skin and mammary gland development and increased gamma-irradiation-induced tumorigenesis in mice carrying a mutation of S1152-ATM phosphorylation site in Brca1.Cancer Res. 2009 Dec 15;69(24):9291-300. doi: 10.1158/0008-5472.CAN-09-2418. Cancer Res. 2009. PMID: 19996295 Free PMC article.
-
Transcription-associated DNA DSBs activate p53 during hiPSC-based neurogenesis.Sci Rep. 2022 Jul 15;12(1):12156. doi: 10.1038/s41598-022-16516-5. Sci Rep. 2022. PMID: 35840793 Free PMC article.
-
Low levels of 3,3'-diindolylmethane activate estrogen receptor α and induce proliferation of breast cancer cells in the absence of estradiol.BMC Cancer. 2014 Jul 21;14:524. doi: 10.1186/1471-2407-14-524. BMC Cancer. 2014. PMID: 25048790 Free PMC article.
References
-
- Bachelier R, Xu X, Wang X, Li W, Naramura M, Gu H, Deng CX (2003) Normal lymphocyte development and thymic lymphoma formation in Brca1 exon-11-deficient mice. Oncogene 22: 528–537 - PubMed
-
- Barlow C, Hirotsune S, Paylor R, Liyanage M, Eckhaus M, Collins F, Shiloh Y, Crawley JN, Ried T, Tagle D, Wynshaw-Boris A (1996) Atm-deficient mice: a paradigm of ataxia telangiectasia. Cell 86: 159–171 - PubMed
-
- Bartek J, Lukas J (2003) Chk1 and Chk2 kinases in checkpoint control and cancer. Cancer Cell 3: 421–429 - PubMed
-
- Bartkova J, Horejsi Z, Koed K, Kramer A, Tort F, Zieger K, Guldberg P, Sehested M, Nesland JM, Lukas C, Orntoft T, Lukas J, Bartek J (2005) DNA damage response as a candidate anti-cancer barrier in early human tumorigenesis. Nature 434: 864–870 - PubMed
-
- Bell DW, Varley JM, Szydlo TE, Kang DH, Wahrer DC, Shannon KE, Lubratovich M, Verselis SJ, Isselbacher KJ, Fraumeni JF, Birch JM, Li FP, Garber JE, Haber DA (1999) Heterozygous germ line hCHK2 mutations in Li-Fraumeni syndrome. Science 286: 2528–2531 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials
Miscellaneous

