Regulation of nuclear factor kappaB in the hippocampus by group I metabotropic glutamate receptors
- PMID: 16672661
- PMCID: PMC6674168
- DOI: 10.1523/JNEUROSCI.4527-05.2006
Regulation of nuclear factor kappaB in the hippocampus by group I metabotropic glutamate receptors
Abstract
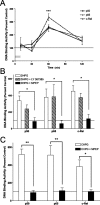
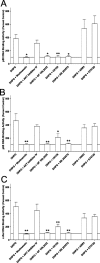
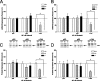
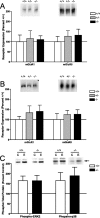
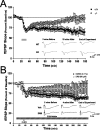
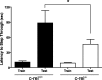
An increasing amount of evidence suggests that the family of nuclear factor kappaB (NF-kappaB) transcription factors plays an important role in synaptic plasticity and long-term memory formation. The present study investigated the regulation of NF-kappaB family members p50, p65/RelA, and c-Rel in the hippocampus in response to metabotropic glutamate receptor (mGluR) signaling. Activation of group I metabotropic glutamate receptors (GpI-mGluRs) with the agonist (S)-3,5-dihydroxyphenylglycine (DHPG) resulted in a time-dependent increase in DNA binding activity of p50, p65, and c-Rel in area CA1 of the hippocampus. An antagonist of mGluR5, 2-Methyl-6-(phenylethynyl)pyridine, inhibited the DHPG-induced activation of NF-kappaB, whereas an antagonist of mGluR1, (S)-(+)-alpha-amino-4-carboxy-2-methylbenzeneacetic acid, did not. Using a series of inhibitors, we investigated the signaling pathways necessary for DHPG-induced activation of NF-kappaB and found that they included the phosphatidyl inositol 3-kinase, protein kinase C, mitogen-activated protein kinase kinase, and p38-mitogen-activated protein kinase pathways. To determine the functional significance of mGluR-induced regulation of NF-kappaB, we measured long-term depression (LTD) of Schaffer-collateral synapses in the hippocampus of c-Rel knock-out mice. Early phase LTD was normal in c-rel(-/-) mice. However, late-phase LTD (>90 min) was impaired in c-rel(-/-) mice. The observations of this deficit in hippocampal synaptic plasticity prompted us to further investigate long-term memory formation in c-rel(-/-) mice. c-rel(-/-) mice exhibited impaired performance in a long-term passive avoidance task, providing additional evidence for c-Rel in long-term memory formation. These results demonstrate that the NF-kappaB transcription factor family is regulated by GpI-mGluRs in the hippocampus and that the c-Rel transcription factor is necessary for long-term maintenance of LTD and formation of long-term memory.
Figures


Similar articles
-
Mechanisms of group I mGluR-dependent long-term depression of NMDA receptor-mediated transmission at Schaffer collateral-CA1 synapses.J Neurophysiol. 2009 Mar;101(3):1375-85. doi: 10.1152/jn.90643.2008. Epub 2008 Dec 24. J Neurophysiol. 2009. PMID: 19109458
-
Sustained activation of metabotropic glutamate receptor 5 and protein tyrosine phosphatases mediate the expression of (S)-3,5-dihydroxyphenylglycine-induced long-term depression in the hippocampal CA1 region.J Neurochem. 2006 Jan;96(1):179-94. doi: 10.1111/j.1471-4159.2005.03527.x. Epub 2005 Nov 8. J Neurochem. 2006. PMID: 16277605
-
Differential roles for group 1 mGluR subtypes in induction and expression of chemically induced hippocampal long-term depression.J Neurophysiol. 2006 Apr;95(4):2427-38. doi: 10.1152/jn.00383.2005. Epub 2006 Jan 18. J Neurophysiol. 2006. PMID: 16421200
-
Metabotropic glutamate receptor-mediated long-term depression: molecular mechanisms.Pharmacol Rev. 2009 Dec;61(4):395-412. doi: 10.1124/pr.109.001735. Epub 2009 Nov 19. Pharmacol Rev. 2009. PMID: 19926678 Free PMC article. Review.
-
Hippocampal metabotropic glutamate receptor long-term depression in health and disease: focus on mitogen-activated protein kinase pathways.J Neurochem. 2016 Oct;139 Suppl 2:200-214. doi: 10.1111/jnc.13592. Epub 2016 May 4. J Neurochem. 2016. PMID: 26923875 Review.
Cited by
-
Silver Nanoparticle-Coated Ethyl Cellulose Inhibits Tumor Necrosis Factor-α of Breast Cancer Cells.Drug Des Devel Ther. 2021 May 13;15:2035-2046. doi: 10.2147/DDDT.S310760. eCollection 2021. Drug Des Devel Ther. 2021. PMID: 34012256 Free PMC article.
-
NEMO reshapes the α-Synuclein aggregate interface and acts as an autophagy adapter by co-condensation with p62.Nat Commun. 2023 Dec 19;14(1):8368. doi: 10.1038/s41467-023-44033-0. Nat Commun. 2023. PMID: 38114471 Free PMC article.
-
Cellular Specificity of NF-κB Function in the Nervous System.Front Immunol. 2019 May 9;10:1043. doi: 10.3389/fimmu.2019.01043. eCollection 2019. Front Immunol. 2019. PMID: 31143184 Free PMC article. Review.
-
The Effect of Simvastatin on the Dynamics of NF-κB-Regulated Neurodegenerative and Neuroprotective Processes in the Acute Phase of Ischemic Stroke.Mol Neurobiol. 2023 Sep;60(9):4935-4951. doi: 10.1007/s12035-023-03371-2. Epub 2023 May 19. Mol Neurobiol. 2023. PMID: 37204689 Free PMC article.
-
MPEP Attenuates Intrahepatic Fat Accumulation in Obese Mice.Int J Mol Sci. 2023 Mar 23;24(7):6076. doi: 10.3390/ijms24076076. Int J Mol Sci. 2023. PMID: 37047048 Free PMC article.
References
-
- Albensi BC, Mattson MP (2000). Evidence for the involvement of TNF and NF-kappaB in hippocampal synaptic plasticity. Synapse 35:151–159. - PubMed
-
- Ambrogi Lorenzini CG, Baldi E, Bucherelli C, Sacchetti B, Tassoni G (1997). Analysis of mnemonic processing by means of totally reversible neural inactivations. Brain Res Brain Res Protoc 1:391–398. - PubMed
-
- Baeuerle PA, Henkel T (1994). Function and activation of NF-kappa B in the immune system. Annu Rev Immunol 12:141–179. - PubMed
-
- Bolshakov VY, Siegelbaum SA (1994). Postsynaptic induction and presynaptic expression of hippocampal long-term depression. Science 264:1148–1152. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials
Miscellaneous
