Pharmacotherapy of acute lung injury and the acute respiratory distress syndrome
- PMID: 16672636
- PMCID: PMC2765330
- DOI: 10.1177/0885066606287045
Pharmacotherapy of acute lung injury and the acute respiratory distress syndrome
Abstract
Acute lung injury and the acute respiratory distress syndrome are common syndromes with a high mortality rate that affect both medical and surgical patients. Better understanding of the pathophysiology of acute lung injury and the acute respiratory distress syndrome and advances in supportive care and mechanical ventilation have led to improved clinical outcomes since the syndrome was first described in 1967. Although several promising pharmacological therapies, including surfactant, nitric oxide, glucocorticoids and lysofylline, have been studied in patients with acute lung injury and the acute respiratory distress syndrome, none of these pharmacological treatments reduced mortality. This article provides an overview of pharmacological therapies of acute lung injury and the acute respiratory distress syndrome tested in clinical trials and current recommendations for their use as well as a discussion of potential future pharmacological therapies including beta(2)-adrenergic agonist therapy, keratinocyte growth factor, and activated protein C.
Figures
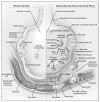
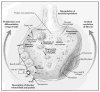
Comment in
-
Adding up the zeros.J Intensive Care Med. 2006 May-Jun;21(3):188-90. doi: 10.1177/0885066606287049. J Intensive Care Med. 2006. PMID: 16672641 No abstract available.
Similar articles
-
Pharmacotherapy for prevention and treatment of acute respiratory distress syndrome: current and experimental approaches.Drugs. 2010 Jul 9;70(10):1255-82. doi: 10.2165/10898570-000000000-00000. Drugs. 2010. PMID: 20568833 Free PMC article. Review.
-
Mechanical ventilation and adjuncts in acute respiratory distress syndrome.Int Anesthesiol Clin. 1997 Winter;35(1):109-24. doi: 10.1097/00004311-199703510-00009. Int Anesthesiol Clin. 1997. PMID: 9113524 Review.
-
Emerging therapies for treatment of acute lung injury and acute respiratory distress syndrome.Expert Opin Emerg Drugs. 2007 Sep;12(3):461-77. doi: 10.1517/14728214.12.3.461. Expert Opin Emerg Drugs. 2007. PMID: 17874973 Review.
-
Surfactant therapy for acute lung injury and acute respiratory distress syndrome.Crit Care Clin. 2011 Jul;27(3):525-59. doi: 10.1016/j.ccc.2011.04.005. Crit Care Clin. 2011. PMID: 21742216 Free PMC article. Review.
-
Exogenous surfactant may improve oxygenation but not mortality in adult patients with acute lung injury/acute respiratory distress syndrome: a meta-analysis of 9 clinical trials.J Cardiothorac Vasc Anesth. 2012 Oct;26(5):849-56. doi: 10.1053/j.jvca.2011.11.006. Epub 2012 Jan 20. J Cardiothorac Vasc Anesth. 2012. PMID: 22265270 Free PMC article.
Cited by
-
CXCR2 in acute lung injury.Mediators Inflamm. 2012;2012:740987. doi: 10.1155/2012/740987. Epub 2012 Jun 6. Mediators Inflamm. 2012. PMID: 22719179 Free PMC article. Review.
-
Programmed cell death and its role in inflammation.Mil Med Res. 2015 May 19;2:12. doi: 10.1186/s40779-015-0039-0. eCollection 2015. Mil Med Res. 2015. PMID: 26045969 Free PMC article.
-
Evaluation of the inflammatory response in a two-hit acute lung injury model using [18F]FDG microPET.Exp Ther Med. 2013 Oct;6(4):894-898. doi: 10.3892/etm.2013.1260. Epub 2013 Aug 13. Exp Ther Med. 2013. PMID: 24137285 Free PMC article.
-
Activated protein C inhalation: a novel therapeutic strategy for acute lung injury.Med Sci Monit. 2011 Jun;17(6):HY11-3. doi: 10.12659/msm.881789. Med Sci Monit. 2011. PMID: 21629195 Free PMC article.
-
Jack of all trades: pleiotropy and the application of chemically modified tetracycline-3 in sepsis and the acute respiratory distress syndrome (ARDS).Pharmacol Res. 2011 Dec;64(6):580-9. doi: 10.1016/j.phrs.2011.06.012. Epub 2011 Jun 21. Pharmacol Res. 2011. PMID: 21767646 Free PMC article. Review.
References
-
- Sackett DL. Rules of evidence and clinical recommendations on the use of antithrombotic agents. Chest. 1986;89 (2suppl):2S–3S. - PubMed
-
- Kopp R, Kuhlen R, Max M, et al. Evidence-based medicine in the therapy of the acute respiratory distress syndrome. Intensive Care Med. 2002;28:244–255. - PubMed
-
- Pulmonary Artery Catheter Consensus conference: consensus statement. Crit Care Med. 1997;25:910–925. - PubMed
-
- Ashbaugh DG, Bigelow DB, Petty TL, et al. Acute respiratory distress in adults. Lancet. 1967;2:319–323. - PubMed
-
- Petty TL, Ashbaugh DG. The adult respiratory distress syndrome. Clinical features, factors influencing prognosis and principles of management. Chest. 1971;60:233–239. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

