Lymphoma cell apoptosis in the liver induced by distant murine cytomegalovirus infection
- PMID: 16641273
- PMCID: PMC1472044
- DOI: 10.1128/JVI.80.10.4801-4819.2006
Lymphoma cell apoptosis in the liver induced by distant murine cytomegalovirus infection
Abstract
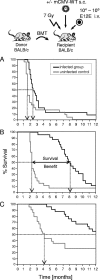
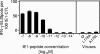
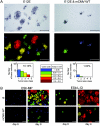
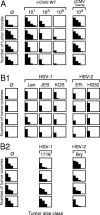
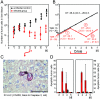
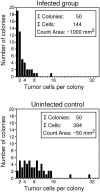
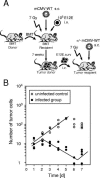
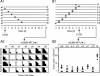
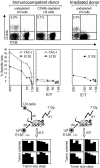
Cytomegalovirus (CMV) poses a threat to the therapy of hematopoietic malignancies by hematopoietic stem cell transplantation, but efficient reconstitution of antiviral immunity prevents CMV organ disease. Tumor relapse originating from a minimal residual leukemia poses another threat. Although a combination of risk factors was supposed to enhance the incidence and severity of transplantation-associated disease, a murine model of a liver-adapted B-cell lymphoma has previously shown a survival benefit and tumor growth inhibition by nonlethal subcutaneous infection with murine CMV. Here we have investigated the underlying antitumoral mechanism. Virus replication proved to be required, since inactivated virions or the highly attenuated enhancerless mutant mCMV-DeltaMIEenh did not impact the lymphoma in the liver. Surprisingly, the dissemination-deficient mutant mCMV-DeltaM36 inhibited tumor growth, even though this virus fails to infect the liver. On the other hand, various strains of herpes simplex viruses consistently failed to control the lymphoma, even though they infect the liver. A quantitative analysis of the tumor growth kinetics identified a transient tumor remission by apoptosis as the antitumoral effector mechanism. Tumor cell colonies with cells surviving the CMV-induced "apoptotic crisis" lead to tumor relapse even in the presence of full-blown tissue infection. Serial transfer of surviving tumor cells did not indicate a selection of apoptosis-resistant genetic variants. NK cell activity of CD49b-expressing cells failed to control the lymphoma upon adoptive transfer. We propose the existence of an innate antitumoral mechanism that is triggered by CMV infection and involves an apoptotic signal effective at a distant site of tumor growth.
Figures
Similar articles
-
Tumor control in a model of bone marrow transplantation and acute liver-infiltrating B-cell lymphoma: an unpredicted novel function of cytomegalovirus.J Virol. 2002 Mar;76(6):2857-70. doi: 10.1128/jvi.76.6.2857-2870.2002. J Virol. 2002. PMID: 11861853 Free PMC article.
-
Mechanism of tumor remission by cytomegalovirus in a murine lymphoma model: evidence for involvement of virally induced cellular interleukin-15.Med Microbiol Immunol. 2015 Jun;204(3):355-66. doi: 10.1007/s00430-015-0408-z. Epub 2015 Mar 25. Med Microbiol Immunol. 2015. PMID: 25805565
-
Hematopoietic cell-mediated dissemination of murine cytomegalovirus is regulated by NK cells and immune evasion.PLoS Pathog. 2021 Jan 28;17(1):e1009255. doi: 10.1371/journal.ppat.1009255. eCollection 2021 Jan. PLoS Pathog. 2021. PMID: 33508041 Free PMC article.
-
[Induction of apoptosis in murine cytomegalovirus infection].Nihon Rinsho. 1998 Jan;56(1):79-82. Nihon Rinsho. 1998. PMID: 9465669 Review. Japanese.
-
Mast cells: innate attractors recruiting protective CD8 T cells to sites of cytomegalovirus infection.Med Microbiol Immunol. 2015 Jun;204(3):327-34. doi: 10.1007/s00430-015-0386-1. Epub 2015 Feb 4. Med Microbiol Immunol. 2015. PMID: 25648117 Review.
Cited by
-
CD8 T-cell-based immunotherapy of cytomegalovirus infection: "proof of concept" provided by the murine model.Med Microbiol Immunol. 2008 Jun;197(2):125-34. doi: 10.1007/s00430-008-0093-2. Epub 2008 Mar 15. Med Microbiol Immunol. 2008. PMID: 18343947 Review.
-
Oncolytic viruses in cancer therapy.Cancer Lett. 2007 Sep 8;254(2):178-216. doi: 10.1016/j.canlet.2007.02.002. Epub 2007 Mar 23. Cancer Lett. 2007. PMID: 17383089 Free PMC article. Review.
-
Tumor control by human cytomegalovirus in a murine model of hepatocellular carcinoma.Mol Ther Oncolytics. 2016 Apr 27;3:16012. doi: 10.1038/mto.2016.12. eCollection 2016. Mol Ther Oncolytics. 2016. PMID: 27626063 Free PMC article.
-
Not just leukemia: CMV may protect against lymphoma recurrence after allogeneic transplant.Leuk Lymphoma. 2017 Apr;58(4):759-761. doi: 10.1080/10428194.2016.1239265. Epub 2016 Oct 12. Leuk Lymphoma. 2017. PMID: 27733072 Free PMC article. No abstract available.
-
From Vaccine Vector to Oncomodulation: Understanding the Complex Interplay between CMV and Cancer.Vaccines (Basel). 2019 Jul 9;7(3):62. doi: 10.3390/vaccines7030062. Vaccines (Basel). 2019. PMID: 31323930 Free PMC article. Review.
References
-
- Almasan, A., and A. Ashkenazi. 2003. Apo2L/TRAIL: apoptosis signaling, biology, and potential for cancer therapy. Cytokine Growth Factor Rev. 14:337-348. - PubMed
-
- Alterio de Goss, M., R. Holtappels, H.-P. Steffens, J. Podlech, P. Angele, L. Dreher, D. Thomas, and M. J. Reddehase. 1998. Control of cytomegalovirus in bone marrow transplantation chimeras lacking the prevailing antigen-presenting molecule in recipient tissues rests primarily on recipient-derived CD8 T cells. J. Virol. 72:7733-7744. - PMC - PubMed
-
- Arase, H., T. Saito, J. H. Phillips, and L. L. Lanier. 2001. Cutting edge: the mouse NK cell-associated antigen recognized by DX5 monoclonal antibody is CD49b (a2 integrin, very late antigen-2). J. Immunol. 167:1141-1144. - PubMed
-
- Aurrand-Lions, M., C. Johnson-Leger, and B. A. Imhof. 2002. The last molecular fortress in leukocyte trans-endothelial migration. Nat. Immunol. 3:116-118. - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases

