Randomized phase II study of concurrent and sequential rituximab and CHOP chemotherapy in untreated indolent B-cell lymphoma
- PMID: 16630123
- PMCID: PMC11159844
- DOI: 10.1111/j.1349-7006.2006.00173.x
Randomized phase II study of concurrent and sequential rituximab and CHOP chemotherapy in untreated indolent B-cell lymphoma
Abstract
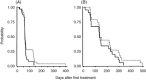
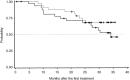
CHOP combined with rituximab (R-CHOP) is regarded as one of the most effective treatments for indolent B-cell non-Hodgkin lymphoma (B-NHL), however, its optimal combination schedule remains unknown. We performed a randomized phase II study to explore a more promising schedule in untreated, advanced indolent B-NHL. Patients were randomized to receive either six courses of CHOP concurrently with rituximab (Arm C), or six courses of CHOP followed by six courses of weekly rituximab (Arm S). A total of 69 patients received the concurrent (n=34) or sequential (n=35) regimen. Overall response rate (ORR) in Arm C was 94% (95% confidence interval [CI], 79 to 99), including a 66% complete response (CR) compared with 97% (95% CI, 85-100), including a 68% CR in Arm S. Patients in Arm C experienced more grade 4 neutropenia (85%versus 70%) and experienced more grade 3 or greater non-hematological toxicities (21%versus 12%). Both arms were tolerated well. With a median follow-up of 28.2 months, the median progression-free survival (PFS) time was 34.2 months in Arm C, and was not reached in Arm S. R-CHOP is highly effective in untreated indolent B-NHL, either concurrent or in a sequential combination. Both combination schedules deserve further investigation.
(Cancer Sci 2006; 97: 305 - 312).
Figures
Similar articles
-
Randomized phase II study of concurrent and sequential combinations of rituximab plus CHOP (cyclophosphamide, doxorubicin, vincristine and prednisolone) chemotherapy in untreated indolent B-cell non-Hodgkin lymphoma: 7-year follow-up results.Cancer Sci. 2010 Dec;101(12):2579-85. doi: 10.1111/j.1349-7006.2010.01703.x. Cancer Sci. 2010. PMID: 20942866 Free PMC article. Clinical Trial.
-
Rituximab: a review of its use in non-Hodgkin's lymphoma and chronic lymphocytic leukaemia.Drugs. 2003;63(8):803-43. doi: 10.2165/00003495-200363080-00005. Drugs. 2003. PMID: 12662126 Review.
-
Rituximab: a review of its use in chronic lymphocytic leukaemia, low-grade or follicular lymphoma and diffuse large B-cell lymphoma.Drugs. 2010 Jul 30;70(11):1445-76. doi: 10.2165/11201110-000000000-00000. Drugs. 2010. PMID: 20614951 Review.
-
Randomized phase II study of biweekly CHOP and dose-escalated CHOP with prophylactic use of lenograstim (glycosylated G-CSF) in aggressive non-Hodgkin's lymphoma: Japan Clinical Oncology Group Study 9505.Ann Oncol. 2002 Sep;13(9):1347-55. doi: 10.1093/annonc/mdf287. Ann Oncol. 2002. PMID: 12196359 Clinical Trial.
-
Long-term update of a phase II study of rituximab in combination with CHOP chemotherapy in patients with previously untreated, aggressive non-Hodgkin's lymphoma.Leuk Lymphoma. 2005 Nov;46(11):1569-73. doi: 10.1080/10428190500217312. Leuk Lymphoma. 2005. PMID: 16236611 Clinical Trial.
Cited by
-
Rituximab in combination with CHOP chemotherapy for the treatment of diffuse large B cell lymphoma in Japan: a retrospective analysis of 1,057 cases from Kyushu Lymphoma Study Group.Int J Hematol. 2010 Mar;91(2):258-66. doi: 10.1007/s12185-009-0475-2. Int J Hematol. 2010. PMID: 20066574
-
Determinants of the optimal first-line therapy for follicular lymphoma: a decision analysis.Am J Hematol. 2010 Apr;85(4):255-60. doi: 10.1002/ajh.21655. Am J Hematol. 2010. PMID: 20196173 Free PMC article.
-
Chemotherapy plus Rituximab versus chemotherapy alone for B-cell non-Hodgkin's lymphoma.Cochrane Database Syst Rev. 2007 Oct 17;2007(4):CD003805. doi: 10.1002/14651858.CD003805.pub2. Cochrane Database Syst Rev. 2007. PMID: 17943799 Free PMC article. Review.
-
Randomized phase II study of concurrent and sequential combinations of rituximab plus CHOP (cyclophosphamide, doxorubicin, vincristine and prednisolone) chemotherapy in untreated indolent B-cell non-Hodgkin lymphoma: 7-year follow-up results.Cancer Sci. 2010 Dec;101(12):2579-85. doi: 10.1111/j.1349-7006.2010.01703.x. Cancer Sci. 2010. PMID: 20942866 Free PMC article. Clinical Trial.
-
Phase II study of oral fludarabine in combination with rituximab for relapsed indolent B-cell non-Hodgkin lymphoma.Cancer Sci. 2009 Oct;100(10):1951-6. doi: 10.1111/j.1349-7006.2009.01247.x. Epub 2009 Jun 17. Cancer Sci. 2009. PMID: 19594547 Free PMC article. Clinical Trial.
References
-
- Rohatiner A, Lister TA. Follicular lymphoma, in Magrath IT (ed.): The Non‐Hodgkin's Lymphomas. London: Oxford University Press, 1997: 867–96.
-
- Berger F, Felman P, Sonet A et al. Nonfollicular small B‐cell lymphomas: a heterogeneous group of patients with distinct clinical features and outcome. Blood 1994; 83: 2829–35. - PubMed
-
- Horning SJ. Natural history of and therapy for the indolent non‐Hodgkin's lymphomas. Semin Oncol 1993; 20: 75–88. - PubMed
-
- Solal‐Celigny PH. Management of histologically indolent non‐Hodgkin's lymphomas. Baillieres Clin Hematol 1996; 9: 669–87. - PubMed
-
- Aisenberg AC. Coherent view of non‐Hodgkin's lymphoma [review]. Clin Oncol 1995; 13: 2656–75. - PubMed
Publication types
MeSH terms
Substances
Supplementary concepts
LinkOut - more resources
Full Text Sources
Research Materials

