Activation of endoplasmic reticulum stress response during the development of ischemic heart disease
- PMID: 16617122
- PMCID: PMC1575464
- DOI: 10.1152/ajpheart.01378.2005
Activation of endoplasmic reticulum stress response during the development of ischemic heart disease
Abstract
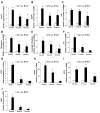
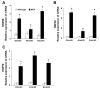
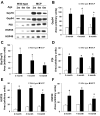
Endoplasmic reticulum (ER) stress has been found to be associated with neurodegenerative diseases and diabetes mellitus. Whether ER stress is involved in the development of heart disease is not known. Cardiac-specific expression of monocyte chemoattractant protein-1 (MCP-1) in mice causes the development of ischemic heart disease. Here we report that microarray analysis of gene expression changes in the heart of these transgenic mice revealed that a cluster of ER stress-related genes was transcriptionally activated in the heart during the development of ischemic heart disease. The gene array results were verified by quantitative real-time PCR that showed highly elevated transcript levels of genes involved in unfolded protein response such as ER and cytoplasmic chaperones, oxidoreductases, protein disulfide isomerase (PDI) family, and ER-associated degradation system such as ubiquitin. Immunoblot analysis confirmed the expression of chaperones, PDI, and ubiquitin. Immunohistochemical analyses showed that ER stress proteins were associated mainly with the degenerating cardiomyocytes. A novel ubiquitin fold modifier (Ufm1) that has not been previously associated with ER stress and not found to be induced under any condition was also found to be upregulated in the hearts of MCP mice (transgenic mice that express MCP-1 specifically in the heart). The present results strongly suggest that activation of ER stress response is involved in the development of ischemic heart disease in this murine model.
Figures



Similar articles
-
Roles for endoplasmic reticulum-associated degradation and the novel endoplasmic reticulum stress response gene Derlin-3 in the ischemic heart.Circ Res. 2010 Feb 5;106(2):307-16. doi: 10.1161/CIRCRESAHA.109.203901. Epub 2009 Nov 25. Circ Res. 2010. PMID: 19940266 Free PMC article.
-
Endoplasmic reticulum stress gene induction and protection from ischemia/reperfusion injury in the hearts of transgenic mice with a tamoxifen-regulated form of ATF6.Circ Res. 2006 May 12;98(9):1186-93. doi: 10.1161/01.RES.0000220643.65941.8d. Epub 2006 Apr 6. Circ Res. 2006. PMID: 16601230
-
Prolonged endoplasmic reticulum stress in hypertrophic and failing heart after aortic constriction: possible contribution of endoplasmic reticulum stress to cardiac myocyte apoptosis.Circulation. 2004 Aug 10;110(6):705-12. doi: 10.1161/01.CIR.0000137836.95625.D4. Epub 2004 Aug 2. Circulation. 2004. PMID: 15289376
-
Biology of endoplasmic reticulum stress in the heart.Circ Res. 2010 Nov 12;107(10):1185-97. doi: 10.1161/CIRCRESAHA.110.227033. Circ Res. 2010. PMID: 21071716 Review.
-
Mechanisms and implications of reactive oxygen species generation during the unfolded protein response: roles of endoplasmic reticulum oxidoreductases, mitochondrial electron transport, and NADPH oxidase.Antioxid Redox Signal. 2009 Oct;11(10):2409-27. doi: 10.1089/ars.2009.2625. Antioxid Redox Signal. 2009. PMID: 19388824 Review.
Cited by
-
The Post-Translational Role of UFMylation in Physiology and Disease.Cells. 2023 Oct 29;12(21):2543. doi: 10.3390/cells12212543. Cells. 2023. PMID: 37947621 Free PMC article. Review.
-
Different Expression of Mitochondrial and Endoplasmic Reticulum Stress Genes in Epicardial Adipose Tissue Depends on Coronary Atherosclerosis.Int J Mol Sci. 2021 Apr 26;22(9):4538. doi: 10.3390/ijms22094538. Int J Mol Sci. 2021. PMID: 33926122 Free PMC article.
-
Monocyte Chemoattractant Protein 1 (MCP-1) in obesity and diabetes.Cytokine. 2012 Oct;60(1):1-12. doi: 10.1016/j.cyto.2012.06.018. Epub 2012 Jul 4. Cytokine. 2012. PMID: 22766373 Free PMC article. Review.
-
The Protective Effect of Lacidipine on Myocardial Remodeling Is Mediated by the Suppression in Expression of GPR78 and CHOP in Rats.Evid Based Complement Alternat Med. 2015;2015:945076. doi: 10.1155/2015/945076. Epub 2015 Jan 21. Evid Based Complement Alternat Med. 2015. PMID: 25688281 Free PMC article.
-
The Myocardial Unfolded Protein Response during Ischemic Cardiovascular Disease.Biochem Res Int. 2012;2012:583170. doi: 10.1155/2012/583170. Epub 2012 Mar 29. Biochem Res Int. 2012. PMID: 22536506 Free PMC article.
References
-
- Beltrami CA, Finato N, Rocco M, Feruglio GA, Puricelli C, Cigola E, Quaini F, Sonnenblick EH, Olivetti G, Anversa P. Structural basis of end-stage failure in ischemic cardiomyopathy in humans. Circulation. 1994;89:151–163. - PubMed
-
- Bernhard G, Karl-Heinz H, Roland HW, Christiane L, Helmut E, Meyerand AK. The cellular oxygen tension regulates expression of the endoplasmic oxidoreductase ERO1-Lα. Eur J Biochem. 2003;270:2228–2235. - PubMed
-
- Brodsky JL, McCracken AA. ER protein quality control and proteasome-mediated protein degradation. Semin Cell Dev Biol. 1999;10:507–513. - PubMed
-
- Bush KT, Goldberg AL, Nigam SK. Proteasome inhibition leads to a heat-shock response, induction of endoplasmic reticulum chaperones, and thermotolerance. J Biol Chem. 1997;272:9086–9092. - PubMed
-
- Chung KKK, Dawson VL, Dawson TM. The role of the ubiquitin-proteasomal pathway in Parkinson’s disease and other neurodegenerative disorders. Trends Neurosci. 2001;24(Suppl 1):S7–S14. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials
Miscellaneous

