Fluorine-substituted cyclofenil derivatives as estrogen receptor ligands: synthesis and structure-affinity relationship study of potential positron emission tomography agents for imaging estrogen receptors in breast cancer
- PMID: 16610793
- PMCID: PMC2522267
- DOI: 10.1021/jm0512037
Fluorine-substituted cyclofenil derivatives as estrogen receptor ligands: synthesis and structure-affinity relationship study of potential positron emission tomography agents for imaging estrogen receptors in breast cancer
Abstract
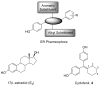
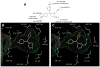
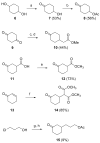
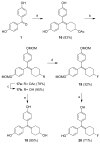
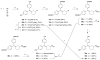
In a search for estrogen receptor (ER) ligands to be radiolabeled with fluorine-18 for imaging of ER-positive breast tumors with positron emission tomography (PET), we investigated cyclofenil analogues substituted at the C3 or C4 position of the cyclohexyl group. McMurry coupling of 4,4'-dihydroxybenzophenone with various ketones produced key cyclofenil intermediates, from which C3 and C4 substituents containing alkyl and various oxygen or fluorine-substituted alkyl groups were elaborated. Binding assays to both ERalpha and ERbeta revealed that the C3 site is more tolerant of steric bulk and polar groups than the C4 site, consistent with a computational model of the ERalpha ligand binding pocket. Fluorine substitution is tolerated very well at some sites, giving some compounds having affinities comparable to or higher than that of estradiol. These fluoro and fluoroalkyl cyclofenils merit further consideration as fluorine-18 labeled ER ligands for PET imaging of ERs in breast tumors.
Figures
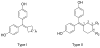
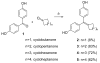

Similar articles
-
Design, synthesis, and evaluation of cyclofenil derivatives for potential SPECT imaging agents.J Biol Inorg Chem. 2010 May;15(4):591-9. doi: 10.1007/s00775-010-0627-0. Epub 2010 Mar 1. J Biol Inorg Chem. 2010. PMID: 20195693
-
Novel Selective Estrogen Receptor Ligand Conjugates Incorporating Endoxifen-Combretastatin and Cyclofenil-Combretastatin Hybrid Scaffolds: Synthesis and Biochemical Evaluation.Molecules. 2017 Aug 31;22(9):1440. doi: 10.3390/molecules22091440. Molecules. 2017. PMID: 28858267 Free PMC article.
-
Synthesis of new carbon-11 labeled cyclofenil derivatives for PET imaging of breast cancer estrogen receptors.Appl Radiat Isot. 2008 Apr;66(4):523-9. doi: 10.1016/j.apradiso.2007.11.006. Epub 2007 Nov 19. Appl Radiat Isot. 2008. PMID: 18155914
-
PET imaging of breast cancer with fluorine-18 radiolabeled estrogens and progestins.Q J Nucl Med. 1998 Mar;42(1):8-17. Q J Nucl Med. 1998. PMID: 9646640 Review.
-
Molecular mechanisms of estrogen action: selective ligands and receptor pharmacology.J Steroid Biochem Mol Biol. 2000 Nov 30;74(5):279-85. doi: 10.1016/s0960-0760(00)00104-7. J Steroid Biochem Mol Biol. 2000. PMID: 11162936 Review.
Cited by
-
Targeting the estrogen receptor with metal-carbonyl derivatives of estradiol.Bioorg Med Chem Lett. 2012 Feb 15;22(4):1670-3. doi: 10.1016/j.bmcl.2011.12.111. Epub 2012 Jan 8. Bioorg Med Chem Lett. 2012. PMID: 22277281 Free PMC article.
-
Design, synthesis, and evaluation of cyclofenil derivatives for potential SPECT imaging agents.J Biol Inorg Chem. 2010 May;15(4):591-9. doi: 10.1007/s00775-010-0627-0. Epub 2010 Mar 1. J Biol Inorg Chem. 2010. PMID: 20195693
-
3D models of MBP, a biologically active metabolite of bisphenol A, in human estrogen receptor α and estrogen receptor β.PLoS One. 2012;7(10):e46078. doi: 10.1371/journal.pone.0046078. Epub 2012 Oct 4. PLoS One. 2012. PMID: 23056236 Free PMC article.
-
Synthesis and biodistribution of fluorine-18-labeled fluorocyclofenils for imaging the estrogen receptor.Nucl Med Biol. 2007 May;34(4):383-90. doi: 10.1016/j.nucmedbio.2007.01.010. Epub 2007 Mar 30. Nucl Med Biol. 2007. PMID: 17499727 Free PMC article.
-
Predictive features of ligand-specific signaling through the estrogen receptor.Mol Syst Biol. 2016 Apr 22;12(4):864. doi: 10.15252/msb.20156701. Mol Syst Biol. 2016. PMID: 27107013 Free PMC article.
References
-
- Katzenellenbogen JA, Katzenellenbogen BS. Nuclear hormone receptors: ligand-activated regulators of transcription and diverse cell responses. Chem Biol. 1996;3:529–536. - PubMed
-
- Turner RT, Rickard D, Spelsberg TC, Sibonga JD. Osteoporosis. Humana Press; 2002. The basic biology of estrogen and bone; pp. 309–330.
-
- Maran A, Turner RT. Effects of estrogen on bone. Recent Research Developments in Endocrinology. 2001:327.
-
- Mendelsohn ME, Karas RH. The protective effects of estrogen on the cardiovascular system. N Engl J Med. 1999;340:1801–1811. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

