Identification of small molecules that induce apoptosis in a Myc-dependent manner and inhibit Myc-driven transformation
- PMID: 16606833
- PMCID: PMC1435363
- DOI: 10.1073/pnas.0601418103
Identification of small molecules that induce apoptosis in a Myc-dependent manner and inhibit Myc-driven transformation
Abstract
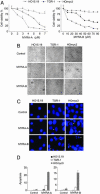
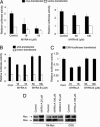
The Myc transcription factor plays a central role in the regulation of cell cycle progression, apoptosis, angiogenesis, and cellular transformation. Myc is a potent oncoprotein that is deregulated in a wide variety of human tumors and is therefore an attractive target for novel cancer therapies. Using a cellular screening approach, we have identified low-molecular-weight compounds, Myc pathway response agents (MYRAs), that induce apoptosis in a c-Myc-dependent manner and inhibit Myc-driven cellular transformation. MYRA-A inhibits Myc transactivation and interferes with the DNA-binding activity of Myc family proteins but has no effect on the E-box-binding protein USF. In contrast, MYRA-B induces Myc-dependent apoptosis without affecting Myc transactivation or Myc/Max DNA binding. Our data show that cellular screening assays can be a powerful strategy for the identification of candidate substances that modulate the Myc pathway. These compounds can be useful tools for studying Myc function and may also be of therapeutic potential as leads for drug development.
Conflict of interest statement
Conflict of interest statement: No conflicts declared.
Figures



Similar articles
-
A selective high affinity MYC-binding compound inhibits MYC:MAX interaction and MYC-dependent tumor cell proliferation.Sci Rep. 2018 Jul 3;8(1):10064. doi: 10.1038/s41598-018-28107-4. Sci Rep. 2018. PMID: 29968736 Free PMC article.
-
Myc overexpression enhances apoptosis induced by small molecules.Cell Cycle. 2006 Oct;5(19):2191-4. doi: 10.4161/cc.5.19.3320. Epub 2006 Oct 1. Cell Cycle. 2006. PMID: 17012843 Review.
-
Stabilizers of the Max homodimer identified in virtual ligand screening inhibit Myc function.Mol Pharmacol. 2009 Sep;76(3):491-502. doi: 10.1124/mol.109.054858. Epub 2009 Jun 4. Mol Pharmacol. 2009. PMID: 19498040 Free PMC article.
-
Computer-aided drug discovery of Myc-Max inhibitors as potential therapeutics for prostate cancer.Eur J Med Chem. 2018 Dec 5;160:108-119. doi: 10.1016/j.ejmech.2018.09.023. Epub 2018 Sep 11. Eur J Med Chem. 2018. PMID: 30326371
-
The Myc oncoprotein as a therapeutic target for human cancer.Semin Cancer Biol. 2006 Aug;16(4):318-30. doi: 10.1016/j.semcancer.2006.07.015. Epub 2006 Aug 3. Semin Cancer Biol. 2006. PMID: 16934487 Review.
Cited by
-
Therapeutic Inhibition of Myc in Cancer. Structural Bases and Computer-Aided Drug Discovery Approaches.Int J Mol Sci. 2018 Dec 29;20(1):120. doi: 10.3390/ijms20010120. Int J Mol Sci. 2018. PMID: 30597997 Free PMC article. Review.
-
Small-Molecule MYC Inhibitors Suppress Tumor Growth and Enhance Immunotherapy.Cancer Cell. 2019 Nov 11;36(5):483-497.e15. doi: 10.1016/j.ccell.2019.10.001. Epub 2019 Oct 31. Cancer Cell. 2019. PMID: 31679823 Free PMC article.
-
Pathological unfoldomics of uncontrolled chaos: intrinsically disordered proteins and human diseases.Chem Rev. 2014 Jul 9;114(13):6844-79. doi: 10.1021/cr400713r. Epub 2014 May 15. Chem Rev. 2014. PMID: 24830552 Free PMC article. Review. No abstract available.
-
Identification of cytotoxic drugs that selectively target tumor cells with MYC overexpression.PLoS One. 2011;6(11):e27988. doi: 10.1371/journal.pone.0027988. Epub 2011 Nov 23. PLoS One. 2011. PMID: 22132187 Free PMC article.
-
A selective high affinity MYC-binding compound inhibits MYC:MAX interaction and MYC-dependent tumor cell proliferation.Sci Rep. 2018 Jul 3;8(1):10064. doi: 10.1038/s41598-018-28107-4. Sci Rep. 2018. PMID: 29968736 Free PMC article.
References
-
- Henriksson M., Lüscher B. Adv. Cancer Res. 1996;68:109–182. - PubMed
-
- Oster S. K., Ho C. S., Soucie E. L., Penn L. Z. Adv. Cancer Res. 2002;84:81–154. - PubMed
-
- Blackwell T. K., Kretzner L., Blackwood E. M., Eisenman R. N., Weintraub H. Science. 1990;250:1149–1151. - PubMed
-
- Lüscher B., Larsson L. G. Oncogene. 1999;18:2955–2966. - PubMed
-
- McMahon S. B., Van Buskirk H. A., Dugan K. A., Copeland T. D., Cole M. D. Cell. 1998;94:363–374. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

