Cytosolic recognition of flagellin by mouse macrophages restricts Legionella pneumophila infection
- PMID: 16606669
- PMCID: PMC1584282
- DOI: 10.1084/jem.20051659
Cytosolic recognition of flagellin by mouse macrophages restricts Legionella pneumophila infection
Abstract
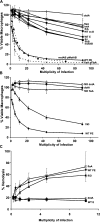
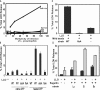
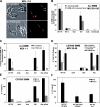
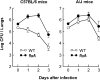
To restrict infection by Legionella pneumophila, mouse macrophages require Naip5, a member of the nucleotide-binding oligomerization domain leucine-rich repeat family of pattern recognition receptors, which detect cytoplasmic microbial products. We report that mouse macrophages restricted L. pneumophila replication and initiated a proinflammatory program of cell death when flagellin contaminated their cytosol. Nuclear condensation, membrane permeability, and interleukin-1beta secretion were triggered by type IV secretion-competent bacteria that encode flagellin. The macrophage response to L. pneumophila was independent of Toll-like receptor signaling but correlated with Naip5 function and required caspase 1 activity. The L. pneumophila type IV secretion system provided only pore-forming activity because listeriolysin O of Listeria monocytogenes could substitute for its contribution. Flagellin monomers appeared to trigger the macrophage response from perforated phagosomes: once heated to disassemble filaments, flagellin triggered cell death but native flagellar preparations did not. Flagellin made L. pneumophila vulnerable to innate immune mechanisms because Naip5+ macrophages restricted the growth of virulent microbes, but flagellin mutants replicated freely. Likewise, after intratracheal inoculation of Naip5+ mice, the yield of L. pneumophila in the lungs declined, whereas the burden of flagellin mutants increased. Accordingly, macrophages respond to cytosolic flagellin by a mechanism that requires Naip5 and caspase 1 to restrict bacterial replication and release proinflammatory cytokines that control L. pneumophila infection.
Figures


Similar articles
-
Global cellular changes induced by Legionella pneumophila infection of bone marrow-derived macrophages.Immunobiology. 2011 Dec;216(12):1274-85. doi: 10.1016/j.imbio.2011.06.008. Epub 2011 Jun 30. Immunobiology. 2011. PMID: 21794945
-
Genetic susceptibility and caspase activation in mouse and human macrophages are distinct for Legionella longbeachae and L. pneumophila.Infect Immun. 2007 Apr;75(4):1933-45. doi: 10.1128/IAI.00025-07. Epub 2007 Jan 29. Infect Immun. 2007. PMID: 17261610 Free PMC article.
-
Flagellin-deficient Legionella mutants evade caspase-1- and Naip5-mediated macrophage immunity.PLoS Pathog. 2006 Mar;2(3):e18. doi: 10.1371/journal.ppat.0020018. Epub 2006 Mar 17. PLoS Pathog. 2006. PMID: 16552444 Free PMC article.
-
[Control of intracellular Legionella pneumophila growth--with special reference to the Lgn1/Naip5/Birc1e gene--].Nihon Saikingaku Zasshi. 2009 Dec;64(2-4):319-30. doi: 10.3412/jsb.64.319. Nihon Saikingaku Zasshi. 2009. PMID: 19628930 Review. Japanese. No abstract available.
-
Naip5/Birc1e and susceptibility to Legionella pneumophila.Trends Microbiol. 2005 Jul;13(7):328-35. doi: 10.1016/j.tim.2005.05.007. Trends Microbiol. 2005. PMID: 15935674 Review.
Cited by
-
NAIP proteins are required for cytosolic detection of specific bacterial ligands in vivo.J Exp Med. 2016 May 2;213(5):657-65. doi: 10.1084/jem.20151809. Epub 2016 Apr 4. J Exp Med. 2016. PMID: 27045008 Free PMC article.
-
Flagellins of Salmonella Typhi and nonpathogenic Escherichia coli are differentially recognized through the NLRC4 pathway in macrophages.J Innate Immun. 2014;6(1):47-57. doi: 10.1159/000351476. Epub 2013 Jun 27. J Innate Immun. 2014. PMID: 23816851 Free PMC article.
-
Mouse macrophages are permissive to motile Legionella species that fail to trigger pyroptosis.Infect Immun. 2010 Jan;78(1):423-32. doi: 10.1128/IAI.00070-09. Epub 2009 Oct 19. Infect Immun. 2010. PMID: 19841075 Free PMC article.
-
A Legionella pneumophila-translocated substrate that is required for growth within macrophages and protection from host cell death.Proc Natl Acad Sci U S A. 2006 Dec 5;103(49):18745-50. doi: 10.1073/pnas.0609012103. Epub 2006 Nov 21. Proc Natl Acad Sci U S A. 2006. PMID: 17124169 Free PMC article.
-
Strategies that modulate inflammasomes: insights from host-pathogen interactions.Semin Immunopathol. 2007 Sep;29(3):261-74. doi: 10.1007/s00281-007-0080-5. Epub 2007 Aug 24. Semin Immunopathol. 2007. PMID: 17717669 Review.
References
-
- Iwasaki, A., and R. Medzhitov. 2004. Toll-like receptor control of the adaptive immune responses. Nat. Immunol. 5:987–995. - PubMed
-
- Inohara, N., M. Chamaillard, C. McDonald, and G. Nunez. 2004. NOD-LRR Proteins: role in host-microbial interactions and inflammatory disease. Annu. Rev. Biochem. 74:355–383. - PubMed
-
- Fortier, A., E. Diez, and P. Gros. 2005. Naip5/Birc1e and susceptibility to Legionella pneumophila. Trends Microbiol. 13:328–335. - PubMed
-
- Diez, E., S.H. Lee, S. Gauthier, Z. Yaraghi, M. Tremblay, S. Vidal, and P. Gros. 2003. Birc1e is the gene within the Lgn1 locus associated with resistance to Legionella pneumophila. Nat. Genet. 33:55–60. - PubMed
-
- Wright, E.K., S.A. Goodart, J.D. Growney, V. Hadinoto, M.G. Endrizzi, E.M. Long, K. Sadigh, A.L. Abney, I. Bernstein-Hanley, and W.F. Dietrich. 2003. Naip5 affects host susceptibility to the intracellular pathogen Legionella pneumophila. Curr. Biol. 13:27–36. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

