Utility of BRAF V600E mutation detection in cytologically indeterminate thyroid nodules
- PMID: 16606457
- PMCID: PMC1481512
- DOI: 10.1186/1742-6413-3-10
Utility of BRAF V600E mutation detection in cytologically indeterminate thyroid nodules
Abstract
Background: Fine needle aspiration (FNA) is widely utilized for evaluation of patients with thyroid nodules. However, approximately 30% are indeterminate for malignancy. Recently, a mutation in the BRAF gene has been reported to be the most common genetic event in papillary thyroid carcinoma (PTC). In this retrospective study, we assessed the utility of BRAF V600E mutation detection for refining indeterminate preoperative cytologic diagnoses in patients with PTC.
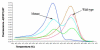
Methods: Archival indeterminate thyroid FNAs and corresponding formalin-fixed, paraffin-embedded (FFPE) surgical samples with PTC were identified in our patient files. DNA extracted from slide scape lysates and 5 mum FFPE sections were evaluated for the BRAF V600E mutation using LightCycler PCR and fluorescent melting curve analysis (LCPCR). Amplification products that showed deviation from the wild-type genomic DNA melting peak, discordant FNA and FFPE matched pairs, and all benign control samples, underwent direct DNA sequencing.
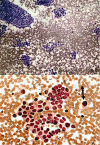
Results: A total of 19 indeterminate thyroid FNAs demonstrating PTC on FFPE surgical samples were included in the study. Using BRAF mutation analysis, the preoperative diagnosis of PTC was confirmed in 3/19 (15.8%) FNA samples that could not be conclusively diagnosed on cytology alone. However, 9/19 (47.4%) FFPE tissue samples were positive for the V600E mutation. Of the discordant pairs, 5/6 FNAs contained less than 50% tumor cells.
Conclusion: When used with indeterminate FNA samples, BRAF mutation analysis may be a useful adjunct technique for confirming the diagnosis of malignancy in an otherwise equivocal case. However, overall tumor cell content of some archival FNA smear slides is a limiting factor for mutation detection.
Figures
Similar articles
-
A morpho-molecular diagnosis of papillary thyroid carcinoma: BRAF V600E detection as an important tool in preoperative evaluation of fine-needle aspirates.Thyroid. 2009 Aug;19(8):837-42. doi: 10.1089/thy.2009.0074. Thyroid. 2009. PMID: 19534623
-
False-negative BRAF V600E mutation results on fine-needle aspiration cytology of papillary thyroid carcinoma.World J Surg Oncol. 2017 Nov 13;15(1):202. doi: 10.1186/s12957-017-1266-5. World J Surg Oncol. 2017. PMID: 29132392 Free PMC article.
-
Development of a Molecular Assay for Detection and Quantification of the BRAF Variation in Residual Tissue From Thyroid Nodule Fine-Needle Aspiration Biopsy Specimens.JAMA Netw Open. 2021 Oct 1;4(10):e2127243. doi: 10.1001/jamanetworkopen.2021.27243. JAMA Netw Open. 2021. PMID: 34613404 Free PMC article.
-
Detection of BRAF V600E activating mutation in papillary thyroid carcinoma using PCR with allele-specific fluorescent probe melting curve analysis.J Clin Pathol. 2007 Nov;60(11):1211-5. doi: 10.1136/jcp.2006.040105. Epub 2007 Feb 13. J Clin Pathol. 2007. PMID: 17298986 Free PMC article. Review.
-
BRAFV600E mutation as a predictor of thyroid malignancy in indeterminate nodules: A systematic review and meta-analysis.Eur J Surg Oncol. 2017 Jul;43(7):1219-1227. doi: 10.1016/j.ejso.2016.11.003. Epub 2016 Nov 22. Eur J Surg Oncol. 2017. PMID: 27923591 Review.
Cited by
-
Molecular testing for somatic mutations improves the accuracy of thyroid fine-needle aspiration biopsy.World J Surg. 2010 Nov;34(11):2589-94. doi: 10.1007/s00268-010-0720-0. World J Surg. 2010. PMID: 20703476 Free PMC article. Clinical Trial.
-
BRAF gene: From human cancers to developmental syndromes.Saudi J Biol Sci. 2015 Jul;22(4):359-73. doi: 10.1016/j.sjbs.2014.10.002. Epub 2014 Oct 23. Saudi J Biol Sci. 2015. PMID: 26150740 Free PMC article. Review.
-
Surgical implications of B-RafV600E mutation in fine-needle aspiration of thyroid nodules.Am J Surg. 2010 Jul;200(1):136-43. doi: 10.1016/j.amjsurg.2009.08.029. Am J Surg. 2010. PMID: 20637346 Free PMC article. Review.
-
The utility of BRAF testing in the management of papillary thyroid cancer.Oncologist. 2010;15(12):1285-93. doi: 10.1634/theoncologist.2010-0156. Epub 2010 Dec 8. Oncologist. 2010. PMID: 21147872 Free PMC article. Review.
-
The Best in CytoJournal: 2006.Cytojournal. 2007 Jun 11;4:12. doi: 10.1186/1742-6413-4-12. Cytojournal. 2007. PMID: 17562005 Free PMC article.
References
-
- Vander JB, Gaston EA, Dawber TR. The significance of nontoxic thyroid nodules. Final report of a 15-year study of the incidence of thyroid malignancy. Ann Intern Med. 1968;69:537–40. - PubMed
-
- Belfiore A, Giuffrida D, La Rosa GL, Ippolito O, Russo G, Fiumara A, et al. High frequency of cancer in cold thyroid nodules occurring at young age. Acta Endocrinol (Copenh) 1989;121:197–202. - PubMed
-
- Jemal A, Tiwari RC, Murray T, Ghafoor A, Samuels A, Ward E, et al. Cancer statistics, 2004. CA Cancer J Clin. 2004;54:8–29. - PubMed
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

