Short-term receptor trafficking in the dorsal vagal complex: an overview
- PMID: 16580267
- PMCID: PMC3062487
- DOI: 10.1016/j.autneu.2006.01.019
Short-term receptor trafficking in the dorsal vagal complex: an overview
Abstract
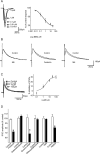
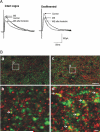
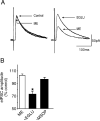
Sensory information from the gastrointestinal (GI) tract is transmitted centrally via primary afferents that terminate within the nucleus of the tractus solitarius (NTS) and utilize glutamate as their major neurotransmitter. Neurons of the NTS integrate this sensory information and transmit it to parasympathetic preganglionic neurons of the dorsal motor nucleus of the vagus (DMV), as well as to other areas, using principally glutamate, GABA and norepinephrine as neurotransmitters. Although susceptible to modulation by a vast array of neurotransmitters, the glutamatergic NTS to DMV synapse seems to play a minor role in the tonic modulation of gastric vagal reflexes. GABAergic neurotransmission between the NTS and DMV, however, is of critical importance as its in vivo blockade induces dramatic effects on gastric tone, motility and secretion. In in vitro experiments, however, this synapse appears initially resistant to modulation by most exogenously applied neuromodulators. Using opioid peptides as a model, this review will discuss the remarkable plasticity of the NTS-DMV GABAergic synapse. Modulation of this synapse appears dependent upon the levels of cAMP within the brainstem circuit. In particular, this review will outline how vagal afferent inputs appear to dampen the cAMP-PKA system via tonic activation of metabotropic glutamate receptors. Removal of vagal sensory input, coincident activation of the cAMP-PKA system, or inhibition of group II metabotropic glutamate receptors, allows receptor trafficking to occur selectively at the level of the NTS-DMV GABAergic synapse. Thus, we propose that the state of activation of vagal sensory inputs determines the gastric motor response via selective engagement of GABAergic synapses. This mini-review is based upon a presentation given at the International Society for Autonomic Neuroscience meeting in Marseille, France in July 2005.
Figures
Similar articles
-
Plasticity of vagal brainstem circuits in the control of gastric function.Neurogastroenterol Motil. 2010 Nov;22(11):1154-63. doi: 10.1111/j.1365-2982.2010.01592.x. Epub 2010 Aug 29. Neurogastroenterol Motil. 2010. PMID: 20804520 Free PMC article.
-
Vagal afferent control of opioidergic effects in rat brainstem circuits.J Physiol. 2006 Sep 15;575(Pt 3):761-76. doi: 10.1113/jphysiol.2006.111104. Epub 2006 Jul 6. J Physiol. 2006. PMID: 16825311 Free PMC article.
-
Nicotine enhances inhibition of mouse vagal motor neurons by modulating excitability of premotor GABAergic neurons in the nucleus tractus solitarii.J Neurophysiol. 2015 Feb 15;113(4):1165-74. doi: 10.1152/jn.00614.2014. Epub 2014 Nov 26. J Neurophysiol. 2015. PMID: 25429117 Free PMC article.
-
Plasticity of vagal brainstem circuits in the control of gastrointestinal function.Auton Neurosci. 2011 Apr 26;161(1-2):6-13. doi: 10.1016/j.autneu.2010.11.001. Epub 2010 Dec 13. Auton Neurosci. 2011. PMID: 21147043 Free PMC article. Review.
-
Inhibitory neurotransmission regulates vagal efferent activity and gastric motility.Exp Biol Med (Maywood). 2016 Jun;241(12):1343-50. doi: 10.1177/1535370216654228. Exp Biol Med (Maywood). 2016. PMID: 27302177 Free PMC article. Review.
Cited by
-
Modulation of inhibitory neurotransmission in brainstem vagal circuits by NPY and PYY is controlled by cAMP levels.Neurogastroenterol Motil. 2009 Dec;21(12):1309-e126. doi: 10.1111/j.1365-2982.2009.01367.x. Epub 2009 Jul 20. Neurogastroenterol Motil. 2009. PMID: 19622099 Free PMC article.
-
The nucleus tractus solitarius: an integrative centre with 'task-matching' capabilities.J Physiol. 2007 Jul 15;582(Pt 2):471. doi: 10.1113/jphysiol.2007.137091. Epub 2007 May 31. J Physiol. 2007. PMID: 17540695 Free PMC article. No abstract available.
-
Vagally mediated effects of glucagon-like peptide 1: in vitro and in vivo gastric actions.J Physiol. 2009 Oct 1;587(Pt 19):4749-59. doi: 10.1113/jphysiol.2009.175067. Epub 2009 Aug 12. J Physiol. 2009. PMID: 19675064 Free PMC article.
-
Mechanisms of Broad-Spectrum Antiemetic Efficacy of Cannabinoids against Chemotherapy-Induced Acute and Delayed Vomiting.Pharmaceuticals (Basel). 2010 Sep 3;3(9):2930-2955. doi: 10.3390/ph3092930. Pharmaceuticals (Basel). 2010. PMID: 27713384 Free PMC article. Review.
-
Characterization of the Basic Membrane Properties of Neurons of the Rat Dorsal Motor Nucleus of the Vagus in Paraquat-Induced Models of Parkinsonism.Neuroscience. 2019 Oct 15;418:122-132. doi: 10.1016/j.neuroscience.2019.08.048. Epub 2019 Sep 3. Neuroscience. 2019. PMID: 31491501 Free PMC article.
References
-
- Andresen MC, Kunze DL. Nucleus tractus solitarius–gateway to neural circulatory control. Annu. Rev. Physiol. 1994;56:93–116. - PubMed
-
- Andresen MC, Yang M. Non-NMDA receptors mediate sensory afferent synaptic transmission in medial nucleus tractus solitarius. Am. J. Physiol. 1990;259:H1307–H1311. - PubMed
-
- Bertolino M, Vicini S, Gillis RA, Travagli RA. Presynaptic α2-adrenoceptors inhibit excitatory synaptic transmission in rat brain stem. Am. J. Physiol. 1997;272:G654–G661. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

