Inhibition of cellular DNA synthesis by the human cytomegalovirus IE86 protein is necessary for efficient virus replication
- PMID: 16571804
- PMCID: PMC1440472
- DOI: 10.1128/JVI.80.8.3872-3883.2006
Inhibition of cellular DNA synthesis by the human cytomegalovirus IE86 protein is necessary for efficient virus replication
Abstract
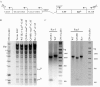
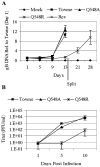
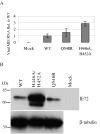
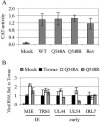
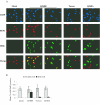
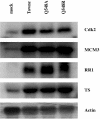
Human cytomegalovirus (HCMV) expresses several proteins that manipulate normal cellular functions, including cellular transcription, apoptosis, immune response, and cell cycle control. The IE2 gene, which is expressed from the HCMV major immediate-early (MIE) promoter, encodes the IE86 protein. IE86 is a multifunctional protein that is essential for viral replication. The functions of IE86 include transactivation of cellular and viral early genes, negative autoregulation of the MIE promoter, induction of cell cycle progression from G0/G1 to G1/S, and arresting cell cycle progression at the G1/S transition in p53-positive human foreskin fibroblast (HFF) cells. Mutations were introduced into the IE2 gene in the context of the viral genome using bacterial artificial chromosomes (BACs). From these HCMV BACs, a recombinant virus (RV) with a single amino acid substitution in the IE86 protein was isolated that replicates slower and to lower titers than wild-type HCMV. HFF cells infected with the Q548R RV undergo cellular DNA synthesis and do not arrest at any point in the cell cycle. The Q548R RV is able to negatively autoregulate the MIE promoter, transactivate viral early genes, activate cellular E2F-responsive genes, and produce infectious virus. This is the first report of a viable recombinant HCMV that is unable to inhibit cellular DNA synthesis in infected HFF cells.
Figures
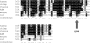

Similar articles
-
The autoregulatory and transactivating functions of the human cytomegalovirus IE86 protein use independent mechanisms for promoter binding.J Virol. 2007 Jun;81(11):5807-18. doi: 10.1128/JVI.02437-06. Epub 2007 Mar 21. J Virol. 2007. PMID: 17376893 Free PMC article.
-
The human cytomegalovirus IE86 protein can block cell cycle progression after inducing transition into the S phase of permissive cells.J Virol. 2000 Aug;74(15):7108-18. doi: 10.1128/jvi.74.15.7108-7118.2000. J Virol. 2000. PMID: 10888651 Free PMC article.
-
Effect of the human cytomegalovirus IE86 protein on expression of E2F-responsive genes: a DNA microarray analysis.Proc Natl Acad Sci U S A. 2002 Mar 5;99(5):2836-41. doi: 10.1073/pnas.052010099. Epub 2002 Feb 26. Proc Natl Acad Sci U S A. 2002. PMID: 11867723 Free PMC article.
-
Functional roles of the human cytomegalovirus essential IE86 protein.Curr Top Microbiol Immunol. 2008;325:133-52. doi: 10.1007/978-3-540-77349-8_8. Curr Top Microbiol Immunol. 2008. PMID: 18637504 Review.
-
Human cytomegalovirus riding the cell cycle.Med Microbiol Immunol. 2015 Jun;204(3):409-19. doi: 10.1007/s00430-015-0396-z. Epub 2015 Mar 17. Med Microbiol Immunol. 2015. PMID: 25776080 Review.
Cited by
-
The human cytomegalovirus major immediate-early proteins as antagonists of intrinsic and innate antiviral host responses.Viruses. 2009 Dec;1(3):760-79. doi: 10.3390/v1030760. Epub 2009 Nov 5. Viruses. 2009. PMID: 21994568 Free PMC article.
-
Neuropathogenesis of congenital cytomegalovirus infection: disease mechanisms and prospects for intervention.Clin Microbiol Rev. 2009 Jan;22(1):99-126, Table of Contents. doi: 10.1128/CMR.00023-08. Clin Microbiol Rev. 2009. PMID: 19136436 Free PMC article. Review.
-
The autoregulatory and transactivating functions of the human cytomegalovirus IE86 protein use independent mechanisms for promoter binding.J Virol. 2007 Jun;81(11):5807-18. doi: 10.1128/JVI.02437-06. Epub 2007 Mar 21. J Virol. 2007. PMID: 17376893 Free PMC article.
-
Casein kinase-2-mediated phosphorylation increases the SUMO-dependent activity of the cytomegalovirus transactivator IE2.J Biol Chem. 2019 Oct 4;294(40):14546-14561. doi: 10.1074/jbc.RA119.009601. Epub 2019 Aug 1. J Biol Chem. 2019. PMID: 31371453 Free PMC article.
-
Biology and pathogenesis of cytomegalovirus in periodontal disease.Periodontol 2000. 2014 Feb;64(1):40-56. doi: 10.1111/j.1600-0757.2012.00448.x. Periodontol 2000. 2014. PMID: 24320955 Free PMC article. Review.
References
-
- Bresnahan, W. A., T. Albrecht, and E. A. Thompson. 1998. The cyclin E promoter is activated by human cytomegalovirus 86-kDa immediate early protein. J. Biol. Chem. 273:22075-22082. - PubMed
-
- Bresnahan, W. A., I. Boldogh, E. A. Thompson, and T. Albrecht. 1996. Human cytomegalovirus inhibits cellular DNA synthesis and arrests productively infected cells in late G1. Virology 224:150-160. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

