Activity and mechanism of action of N-methanocarbathymidine against herpesvirus and orthopoxvirus infections
- PMID: 16569849
- PMCID: PMC1426929
- DOI: 10.1128/AAC.50.4.1336-1341.2006
Activity and mechanism of action of N-methanocarbathymidine against herpesvirus and orthopoxvirus infections
Abstract
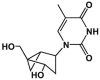
N-Methanocarbathymidine [(N)-MCT] is a conformationally locked nucleoside analog that is active against some herpesviruses and orthopoxviruses in vitro. The antiviral activity of this molecule is dependent on the type I thymidine kinase (TK) in herpes simplex virus and also appears to be dependent on the type II TK expressed by cowpox and vaccinia viruses, suggesting that it is a substrate for both of these divergent forms of the enzyme. The drug is also a good inhibitor of viral DNA synthesis in both viruses and is consistent with inhibition of the viral DNA polymerase once it is activated by the viral TK homologs. This mechanism of action explains the rather unusual spectrum of activity, which is limited to orthopoxviruses, alphaherpesviruses, and Epstein-Barr virus, since these viruses express molecules with TK activity that can phosphorylate and thus activate the drug. The compound is also effective in vivo and reduces the mortality of mice infected with orthopoxviruses, as well as those infected with herpes simplex virus type 1 when treatment is initiated 24 h after infection. These results indicate that (N)-MCT is active in vitro and in vivo, and its mechanism of action suggests that the molecule may be an effective therapeutic for orthopoxvirus and herpesvirus infections, thus warranting further development.
Figures

Similar articles
-
Cell line dependency for antiviral activity and in vivo efficacy of N-methanocarbathymidine against orthopoxvirus infections in mice.Antiviral Res. 2007 Jan;73(1):69-77. doi: 10.1016/j.antiviral.2006.04.010. Epub 2006 May 5. Antiviral Res. 2007. PMID: 16712967
-
Antiherpesvirus activities of two novel 4'-thiothymidine derivatives, KAY-2-41 and KAH-39-149, are dependent on viral and cellular thymidine kinases.Antimicrob Agents Chemother. 2014 Aug;58(8):4328-40. doi: 10.1128/AAC.02825-14. Epub 2014 May 12. Antimicrob Agents Chemother. 2014. PMID: 24820089 Free PMC article.
-
Activities of certain 5-substituted 4'-thiopyrimidine nucleosides against orthopoxvirus infections.Antimicrob Agents Chemother. 2009 Feb;53(2):572-9. doi: 10.1128/AAC.01257-08. Epub 2008 Nov 24. Antimicrob Agents Chemother. 2009. PMID: 19029322 Free PMC article.
-
The history of N-methanocarbathymidine: the investigation of a conformational concept leads to the discovery of a potent and selective nucleoside antiviral agent.Antiviral Res. 2006 Sep;71(2-3):268-75. doi: 10.1016/j.antiviral.2006.04.012. Epub 2006 May 6. Antiviral Res. 2006. PMID: 16730077 Review.
-
Thymidine kinase and protein kinase in drug-resistant herpesviruses: Heads of a Lernaean Hydra.Drug Resist Updat. 2018 Mar;37:1-16. doi: 10.1016/j.drup.2018.01.003. Epub 2018 Jan 31. Drug Resist Updat. 2018. PMID: 29548479 Review.
Cited by
-
Introduction of a cyano group at the 2-position of an (R,S)-3-hydroxy-2-(phosphonomethoxy)propyl (HPMP) derivative of thymine elicits selective anti-HBV activity.RSC Med Chem. 2021 Apr 29;12(5):804-808. doi: 10.1039/d1md00086a. eCollection 2021 May 26. RSC Med Chem. 2021. PMID: 34124679 Free PMC article.
-
Efficacy of N-methanocarbathymidine against genital herpes simplex virus type 2 shedding and infection in guinea pigs.Antivir Chem Chemother. 2015 Feb;24(1):19-27. doi: 10.1177/2040206614566581. Antivir Chem Chemother. 2015. PMID: 26149263 Free PMC article.
-
Inhibition of herpesvirus replication by 5-substituted 4'-thiopyrimidine nucleosides.Antimicrob Agents Chemother. 2009 Dec;53(12):5251-8. doi: 10.1128/AAC.00417-09. Epub 2009 Sep 21. Antimicrob Agents Chemother. 2009. PMID: 19770274 Free PMC article.
-
Orthopoxvirus targets for the development of new antiviral agents.Antiviral Res. 2012 May;94(2):111-25. doi: 10.1016/j.antiviral.2012.02.012. Epub 2012 Mar 8. Antiviral Res. 2012. PMID: 22406470 Free PMC article. Review.
-
Synthesis and antiviral activities of methylenecyclopropane analogs with 6-alkoxy and 6-alkylthio substitutions that exhibit broad-spectrum antiviral activity against human herpesviruses.Antimicrob Agents Chemother. 2013 Aug;57(8):3518-27. doi: 10.1128/AAC.00429-13. Epub 2013 May 13. Antimicrob Agents Chemother. 2013. PMID: 23669381 Free PMC article.
References
-
- Ali, A. N., P. C. Turner, M. A. Brooks, and R. W. Moyer. 1994. The SPI-1 gene of rabbitpox virus determines host range and is required for hemorrhagic pock formation. Virology 202:305-314. - PubMed
-
- Biron, K. K., J. A. Fyfe, J. E. Noblin, and G. B. Elion. 1982. Selection and preliminary characterization of acyclovir-resistant mutants of varicella zoster virus. Am. J. Med. 73:383-386. - PubMed
-
- Black, M. E., and D. E. Hruby. 1992. A single amino acid substitution abolishes feedback inhibition of vaccinia virus thymidine kinase. J. Biol. Chem. 267:9743-9748. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

