The ESCRT-III subunit hVps24 is required for degradation but not silencing of the epidermal growth factor receptor
- PMID: 16554368
- PMCID: PMC1474783
- DOI: 10.1091/mbc.e05-10-0915
The ESCRT-III subunit hVps24 is required for degradation but not silencing of the epidermal growth factor receptor
Abstract
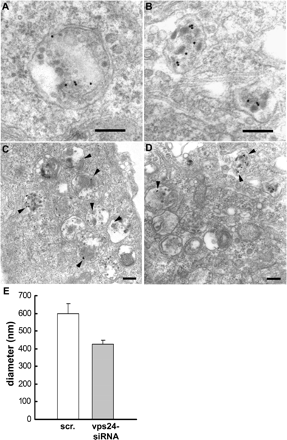
The endosomal sorting complexes required for transport, ESCRT-I, -II, and -III, are thought to mediate the biogenesis of multivesicular endosomes (MVEs) and endosomal sorting of ubiquitinated membrane proteins. Here, we have compared the importance of the ESCRT-I subunit tumor susceptibility gene 101 (Tsg101) and the ESCRT-III subunit hVps24/CHMP3 for endosomal functions and receptor signaling. Like Tsg101, endogenous hVps24 localized mainly to late endosomes. Depletion of hVps24 by siRNA showed that this ESCRT subunit, like Tsg101, is important for degradation of the epidermal growth factor (EGF) receptor (EGFR) and for transport of the receptor from early endosomes to lysosomes. Surprisingly, however, whereas depletion of Tsg101 caused sustained EGF activation of the mitogen-activated protein kinase pathway, depletion of hVps24 had no such effect. Moreover, depletion of Tsg101 but not of hVps24 caused a major fraction of internalized EGF to accumulate in nonacidified endosomes. Electron microscopy of hVps24-depleted cells showed an accumulation of EGFRs in MVEs that were significantly smaller than those in control cells, probably because of an impaired fusion with lyso-bisphosphatidic acid-positive late endosomes/lysosomes. Together, our results reveal functional differences between ESCRT-I and ESCRT-III in degradative protein trafficking and indicate that degradation of the EGFR is not required for termination of its signaling.
Figures





Similar articles
-
RILP is required for the proper morphology and function of late endosomes.J Cell Sci. 2007 Nov 1;120(Pt 21):3729-37. doi: 10.1242/jcs.017301. J Cell Sci. 2007. PMID: 17959629
-
Vps22/EAP30 in ESCRT-II mediates endosomal sorting of growth factor and chemokine receptors destined for lysosomal degradation.Traffic. 2007 Nov;8(11):1617-29. doi: 10.1111/j.1600-0854.2007.00630.x. Epub 2007 Aug 20. Traffic. 2007. PMID: 17714434
-
Multivesicular endosome biogenesis in the absence of ESCRTs.Traffic. 2009 Jul;10(7):925-37. doi: 10.1111/j.1600-0854.2009.00920.x. Epub 2009 Apr 10. Traffic. 2009. PMID: 19490536
-
Roles for ER:endosome membrane contact sites in ligand-stimulated intraluminal vesicle formation.Biochem Soc Trans. 2018 Oct 19;46(5):1055-1062. doi: 10.1042/BST20170432. Epub 2018 Sep 20. Biochem Soc Trans. 2018. PMID: 30242114 Free PMC article. Review.
-
ESCRT proteins in physiology and disease.Exp Cell Res. 2009 May 15;315(9):1619-26. doi: 10.1016/j.yexcr.2008.10.013. Epub 2008 Oct 28. Exp Cell Res. 2009. PMID: 19013455 Review.
Cited by
-
Spastin and ESCRT-III coordinate mitotic spindle disassembly and nuclear envelope sealing.Nature. 2015 Jun 11;522(7555):231-5. doi: 10.1038/nature14408. Epub 2015 Jun 3. Nature. 2015. PMID: 26040712
-
Endosome maturation.EMBO J. 2011 Aug 31;30(17):3481-500. doi: 10.1038/emboj.2011.286. EMBO J. 2011. PMID: 21878991 Free PMC article. Review.
-
Role of EGF Receptor Regulatory Networks in the Host Response to Viral Infections.Front Cell Infect Microbiol. 2022 Jan 10;11:820355. doi: 10.3389/fcimb.2021.820355. eCollection 2021. Front Cell Infect Microbiol. 2022. PMID: 35083168 Free PMC article. Review.
-
Sorting of EGF and transferrin at the plasma membrane and by cargo-specific signaling to EEA1-enriched endosomes.J Cell Sci. 2008 Oct 15;121(Pt 20):3445-58. doi: 10.1242/jcs.031484. Epub 2008 Sep 30. J Cell Sci. 2008. PMID: 18827013 Free PMC article.
-
De Novo VPS4A Mutations Cause Multisystem Disease with Abnormal Neurodevelopment.Am J Hum Genet. 2020 Dec 3;107(6):1129-1148. doi: 10.1016/j.ajhg.2020.10.012. Epub 2020 Nov 12. Am J Hum Genet. 2020. PMID: 33186545 Free PMC article.
References
-
- Authier F., Mort J. S., Bell A. W., Posner B. I., Bergeron J. J. Proteolysis of glucagon within hepatic endosomes by membrane-associated cathepsins B and D. J. Biol. Chem. 1995;270:15798–15807. - PubMed
-
- Babst M., Katzmann D. J., Estepa-Sabal E. J., Meerloo T., Emr S. D. Escrt-III: an endosome-associated heterooligomeric protein complex required for mvb sorting. Dev. Cell. 2002a;3:271–282. - PubMed
-
- Babst M., Katzmann D. J., Snyder W. B., Wendland B., Emr S. D. Endosome-associated complex, ESCRT-II, recruits transport machinery for protein sorting at the multivesicular body. Dev. Cell. 2002b;3:283–289. - PubMed
-
- Babst M., Odorizzi G., Estepa E. J., Emr S. D. Mammalian tumor susceptibility gene 101 (TSG101) and the yeast homologue, Vps23p, both function in late endosomal trafficking. Traffic. 2000;1:248–258. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials
Miscellaneous

