Pore-forming and enzymatic activities of Bordetella pertussis adenylate cyclase toxin synergize in promoting lysis of monocytes
- PMID: 16552051
- PMCID: PMC1418931
- DOI: 10.1128/IAI.74.4.2207-2214.2006
Pore-forming and enzymatic activities of Bordetella pertussis adenylate cyclase toxin synergize in promoting lysis of monocytes
Abstract
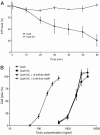
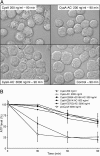
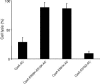
Bordetella adenylate cyclase (AC) toxin-hemolysin (CyaA) targets myeloid phagocytes expressing the alphaMbeta2 integrin (CD11b/CD18) and delivers into their cytosol an AC enzyme that converts ATP into cyclic AMP (cAMP). In parallel, CyaA acts as a hemolysin, forming small membrane pores. Using specific mutations, we dissected the contributions of the two activities to cytolytic potency of CyaA on J774A.1 murine monocytes. The capacity of AC to penetrate cells and deplete cytosolic ATP was essential for promoting lysis and the enzymatically inactive but fully hemolytic CyaA-AC- toxoid exhibited a 15-fold-lower cytolytic capacity on J774A.1 cells than intact CyaA. Moreover, a two- or fourfold drop of specific hemolytic activity of the CyaA-E570Q and CyaA-E581P mutants was overpowered by an intact capacity to dissipate cytosolic ATP into cAMP, allowing the less hemolytic proteins to promote lysis of J774A.1 cells as efficiently as intact CyaA. However, an increased hemolytic activity, due to lysine substitutions of glutamates 509, 516, and 581 in the pore-forming domain, conferred on AC- toxoids a correspondingly enhanced cytolytic potency. Moreover, a threefold increase in hemolytic activity could override a fourfold drop in capacity to convert cellular ATP to cAMP, conferring on the CyaA-E581K construct an overall twofold increased cytolytic potency. Hence, although appearing auxiliary in cytolytic action of the toxin on nucleated cells, the pore-forming activity can synergize with ATP-depleting activity of the cell-invasive AC enzyme and complement its action toward maximal cytotoxicity.
Figures


Similar articles
-
Acylation of lysine 860 allows tight binding and cytotoxicity of Bordetella adenylate cyclase on CD11b-expressing cells.Biochemistry. 2005 Sep 27;44(38):12759-66. doi: 10.1021/bi050459b. Biochemistry. 2005. PMID: 16171390
-
Cyclic AMP-Elevating Capacity of Adenylate Cyclase Toxin-Hemolysin Is Sufficient for Lung Infection but Not for Full Virulence of Bordetella pertussis.Infect Immun. 2017 May 23;85(6):e00937-16. doi: 10.1128/IAI.00937-16. Print 2017 Jun. Infect Immun. 2017. PMID: 28396322 Free PMC article.
-
Third activity of Bordetella adenylate cyclase (AC) toxin-hemolysin. Membrane translocation of AC domain polypeptide promotes calcium influx into CD11b+ monocytes independently of the catalytic and hemolytic activities.J Biol Chem. 2007 Feb 2;282(5):2808-20. doi: 10.1074/jbc.M609979200. Epub 2006 Dec 4. J Biol Chem. 2007. PMID: 17148436
-
Bordetella adenylate cyclase toxin: a unique combination of a pore-forming moiety with a cell-invading adenylate cyclase enzyme.Pathog Dis. 2015 Nov;73(8):ftv075. doi: 10.1093/femspd/ftv075. Epub 2015 Sep 20. Pathog Dis. 2015. PMID: 26391732 Free PMC article. Review.
-
Structure-Function Relationships Underlying the Capacity of Bordetella Adenylate Cyclase Toxin to Disarm Host Phagocytes.Toxins (Basel). 2017 Sep 24;9(10):300. doi: 10.3390/toxins9100300. Toxins (Basel). 2017. PMID: 28946636 Free PMC article. Review.
Cited by
-
Delivery of large heterologous polypeptides across the cytoplasmic membrane of antigen-presenting cells by the Bordetella RTX hemolysin moiety lacking the adenylyl cyclase domain.Infect Immun. 2012 Mar;80(3):1181-92. doi: 10.1128/IAI.05711-11. Epub 2012 Jan 3. Infect Immun. 2012. PMID: 22215742 Free PMC article.
-
Selective Enhancement of the Cell-Permeabilizing Activity of Adenylate Cyclase Toxin Does Not Increase Virulence of Bordetella pertussis.Int J Mol Sci. 2021 Oct 28;22(21):11655. doi: 10.3390/ijms222111655. Int J Mol Sci. 2021. PMID: 34769101 Free PMC article.
-
Membrane-Pore Forming Characteristics of the Bordetella pertussis CyaA-Hemolysin Domain.Toxins (Basel). 2015 Apr 30;7(5):1486-96. doi: 10.3390/toxins7051486. Toxins (Basel). 2015. PMID: 25941766 Free PMC article.
-
Passively released heme from hemoglobin and myoglobin is a potential source of nutrient iron for Bordetella bronchiseptica.Infect Immun. 2007 Oct;75(10):4857-66. doi: 10.1128/IAI.00407-07. Epub 2007 Jul 30. Infect Immun. 2007. PMID: 17664260 Free PMC article.
-
Bioengineering of Bordetella pertussis Adenylate Cyclase Toxin for Antigen-Delivery and Immunotherapy.Toxins (Basel). 2018 Jul 20;10(7):302. doi: 10.3390/toxins10070302. Toxins (Basel). 2018. PMID: 30037010 Free PMC article. Review.
References
-
- Bachelet, M., M. J. Richard, D. Francois, and B. S. Polla. 2002. Mitochondrial alterations precede Bordetella pertussis-induced apoptosis. FEMS Immunol. Med. Microbiol. 32:125-131. - PubMed
-
- Basar, T., V. Havlicek, S. Bezouskova, M. Hackett, and P. Sebo. 2001. Acylation of lysine 983 is sufficient for toxin activity of Bordetella pertussis adenylate cyclase. Substitutions of alanine 140 modulate acylation site selectivity of the toxin acyltransferase CyaC. J. Biol. Chem. 276:348-354. - PubMed
-
- Basar, T., V. Havlicek, S. Bezouskova, P. Halada, M. Hackett, and P. Sebo. 1999. The conserved lysine 860 in the additional fatty-acylation site of Bordetella pertussis adenylate cyclase toxin is crucial for toxin function independently of its acylation status. J. Biol. Chem. 274:10777-10783. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

