Differential effects of bryostatin 1 and 12-O-tetradecanoylphorbol-13-acetate on the regulation and activation of RasGRP1 in mouse epidermal keratinocytes
- PMID: 16546974
- PMCID: PMC1885540
- DOI: 10.1158/1535-7163.MCT-05-0317
Differential effects of bryostatin 1 and 12-O-tetradecanoylphorbol-13-acetate on the regulation and activation of RasGRP1 in mouse epidermal keratinocytes
Abstract
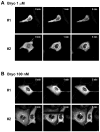
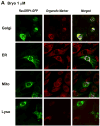
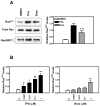
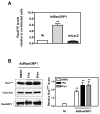
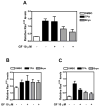
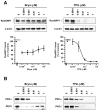
The antitumor agent bryostatin 1 and the tumor-promoting phorbol esters function as structural mimetics of the second lipid messenger diacylglycerol (DAG) by binding to the C1 domain of DAG receptors. However, bryostatin 1 and the phorbol esters often differ in their cellular actions. In mouse skin, the phorbol ester 12-O-tetradecanoylphorbol-13-acetate (TPA) is a potent tumor promoter, whereas bryostatin 1 lacks this activity and antagonizes the tumor-promoting effects of TPA. Although protein kinase C mediates many of the effects of DAG on skin, the exact mechanisms responsible for the biology of bryostatin 1 and TPA in the epidermis have not been elucidated. We recently reported that the novel DAG receptor RasGRP1 is expressed in mouse keratinocytes and mediates TPA-induced Ras activation. This finding prompted us to examine the regulation of RasGRP1 by bryostatin 1. We found that whereas TPA induced translocation of RasGRP1 to both the plasma and internal membranes of the keratinocytes, bryostatin 1 recruited RasGRP1 only to internal membranes and the nuclear envelope. In addition, TPA led to a concentration-dependent down-regulation of RasGRP1, whereas bryostatin 1 failed to induce full RasGRP1 down-regulation. Interestingly, bryostatin 1 was less effective than TPA at activating Ras. The results presented here suggest the possibility that a differential modulation of RasGRP1 by bryostatin 1 compared with TPA could participate in the disparate responses of the epidermal cells to both DAG analogues. This result may have implications in the understanding of the antitumor effects of bryostatin 1 in the skin.
Figures




Similar articles
-
A Focused Review of Ras Guanine Nucleotide-Releasing Protein 1 in Immune Cells and Cancer.Int J Mol Sci. 2023 Jan 13;24(2):1652. doi: 10.3390/ijms24021652. Int J Mol Sci. 2023. PMID: 36675167 Free PMC article. Review.
-
RasGRP1 is essential for ras activation by the tumor promoter 12-O-tetradecanoylphorbol-13-acetate in epidermal keratinocytes.J Biol Chem. 2010 May 21;285(21):15724-30. doi: 10.1074/jbc.M109.100016. Epub 2010 Mar 22. J Biol Chem. 2010. PMID: 20308057 Free PMC article.
-
RasGRP1 represents a novel non-protein kinase C phorbol ester signaling pathway in mouse epidermal keratinocytes.J Biol Chem. 2003 Dec 26;278(52):52792-801. doi: 10.1074/jbc.M308240200. Epub 2003 Oct 7. J Biol Chem. 2003. PMID: 14532295
-
Synthetic bryostatin analogues activate the RasGRP1 signaling pathway.J Med Chem. 2004 Dec 16;47(26):6638-44. doi: 10.1021/jm0495069. J Med Chem. 2004. PMID: 15588099
-
Exploring the multifaceted role of RASGRP1 in disease: immune, neural, metabolic, and oncogenic perspectives.Cell Cycle. 2024 Mar;23(6):722-746. doi: 10.1080/15384101.2024.2366009. Epub 2024 Jun 12. Cell Cycle. 2024. PMID: 38865342 Review.
Cited by
-
A Focused Review of Ras Guanine Nucleotide-Releasing Protein 1 in Immune Cells and Cancer.Int J Mol Sci. 2023 Jan 13;24(2):1652. doi: 10.3390/ijms24021652. Int J Mol Sci. 2023. PMID: 36675167 Free PMC article. Review.
-
RasGRP1 overexpression in the epidermis of transgenic mice contributes to tumor progression during multistage skin carcinogenesis.Cancer Res. 2007 Nov 1;67(21):10190-7. doi: 10.1158/0008-5472.CAN-07-2375. Cancer Res. 2007. PMID: 17974959 Free PMC article.
-
RasGRP1 is essential for ras activation by the tumor promoter 12-O-tetradecanoylphorbol-13-acetate in epidermal keratinocytes.J Biol Chem. 2010 May 21;285(21):15724-30. doi: 10.1074/jbc.M109.100016. Epub 2010 Mar 22. J Biol Chem. 2010. PMID: 20308057 Free PMC article.
-
RasGRP1 transgenic mice develop cutaneous squamous cell carcinomas in response to skin wounding: potential role of granulocyte colony-stimulating factor.Am J Pathol. 2009 Jul;175(1):392-9. doi: 10.2353/ajpath.2009.090036. Epub 2009 Jun 4. Am J Pathol. 2009. PMID: 19497993 Free PMC article.
-
Targeted deletion of RasGRP1 impairs skin tumorigenesis.Carcinogenesis. 2014 May;35(5):1084-91. doi: 10.1093/carcin/bgu016. Epub 2014 Jan 24. Carcinogenesis. 2014. PMID: 24464785 Free PMC article.
References
-
- Pettit GR, Herald CL, Doubek DL, Herald DL, Arnold E, Clardy J. Isolation and structure of bryostatin 1. J Am Chem Soc. 1982;104:6846–8.
-
- Kazanietz MG, Bustelo XR, Barbacid M, Kolch W, Mischak H, Wong G, et al. Zinc finger domains and phorbol ester pharmacophore. Analysis of binding to mutated form of protein kinase C zeta and the vav and c-raf proto-oncogene products. J Biol Chem. 1994 Apr 15;269(15):11590–4. - PubMed
-
- Hennings H, Blumberg PM, Pettit GR, Herald CL, Shores R, Yuspa SH. Bryostatin 1, an activator of protein kinase C, inhibits tumor promotion by phorbol esters in SENCAR mouse skin. Carcinogenesis. 1987 Sep;8(9):1343–6. - PubMed
-
- Newton AC. Protein Kinase C: Structure, Function, and Regulation. J Biol Chem. 1995 December 1;270(48):28495–8. - PubMed
-
- Szallasi Z, Du L, Levine R, Lewin N, Nguyen P, Williams M, et al. The bryostatins inhibit growth of B16/F10 melanoma cells in vitro through a protein kinase C-independent mechanism: dissociation of activities using 26-epi-bryostatin 1. Cancer Res. 1996 May 1;56(9):2105–11. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

