Regulatory mechanisms and functions of intermediate filaments: a study using site- and phosphorylation state-specific antibodies
- PMID: 16542212
- PMCID: PMC11159468
- DOI: 10.1111/j.1349-7006.2006.00161.x
Regulatory mechanisms and functions of intermediate filaments: a study using site- and phosphorylation state-specific antibodies
Abstract
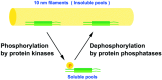
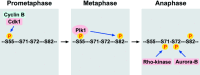
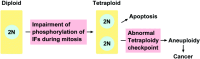
Intermediate filaments (IF) form the structural framework of the cytoskeleton. Although histopathological detection of IF proteins is utilized for examining cancer specimens as reliable markers, the molecular mechanisms by which IF are involved in the biology of cancer cells are still unclear. We found that site-specific phosphorylation of IF proteins induces the disassembly of filament structures. To further dissect the in vivo spatiotemporal dynamics of IF phosphorylation, we developed site- and phosphorylation state-specific antibodies. Using these antibodies, we detected kinase activities that specifically phosphorylate type III IF, including vimentin, glial fibrillary acidic protein and desmin, during mitosis. Cdk1 phosphorylates vimentin-Ser55 from prometaphase to metaphase, leading to the recruitment of Polo-like kinase 1 (Plk1) to vimentin. Upon binding to Phospho-Ser55 of vimentin, Plk1 is activated, and then phosphorylates vimentin-Ser82. During cytokinesis, Rho-kinase and Aurora-B specifically phosphorylate IF at the cleavage furrow. IF phosphorylation by Cdk1, Plk1, Rho-kinase and Aurora-B plays an important role in the local IF breakdown, and is essential for the efficient segregation of IF networks into daughter cells. As another part of our research on IF, we have set out to find the binding partners with simple epithelial keratin 8/18. We identified tumor necrosis factor receptor type 1-associated death domain protein (TRADD) as a keratin 18-binding protein. Together with data from other laboratories, it is proposed that simple epithelial keratins may play a role in modulating the response to some apoptotic signals. Elucidation of the precise molecular functions of IF is expected to improve our understanding of tumor development, invasion and metastasis.
Figures

Similar articles
-
Phosphorylation by Cdk1 induces Plk1-mediated vimentin phosphorylation during mitosis.J Cell Biol. 2005 Nov 7;171(3):431-6. doi: 10.1083/jcb.200504091. Epub 2005 Oct 31. J Cell Biol. 2005. PMID: 16260496 Free PMC article.
-
Regulation of keratin 5/14 intermediate filaments by CDK1, Aurora-B, and Rho-kinase.Biochem Biophys Res Commun. 2018 Apr 6;498(3):544-550. doi: 10.1016/j.bbrc.2018.03.016. Epub 2018 Mar 6. Biochem Biophys Res Commun. 2018. PMID: 29518391
-
Functional significance of the specific sites phosphorylated in desmin at cleavage furrow: Aurora-B may phosphorylate and regulate type III intermediate filaments during cytokinesis coordinatedly with Rho-kinase.Mol Biol Cell. 2003 Apr;14(4):1489-500. doi: 10.1091/mbc.e02-09-0612. Mol Biol Cell. 2003. PMID: 12686604 Free PMC article.
-
Cytoskeleton-associated proteins: their role as cellular integrators in the neoplastic process.Crit Rev Oncol Hematol. 1985;3(3):191-204. doi: 10.1016/s1040-8428(85)80026-3. Crit Rev Oncol Hematol. 1985. PMID: 2412718 Review.
-
Regulation of intermediate filament organization during cytokinesis: possible roles of Rho-associated kinase.Microsc Res Tech. 2000 Apr 15;49(2):173-82. doi: 10.1002/(SICI)1097-0029(20000415)49:2<173::AID-JEMT10>3.0.CO;2-A. Microsc Res Tech. 2000. PMID: 10816257 Review.
Cited by
-
Type I keratin 17 protein is phosphorylated on serine 44 by p90 ribosomal protein S6 kinase 1 (RSK1) in a growth- and stress-dependent fashion.J Biol Chem. 2011 Dec 9;286(49):42403-42413. doi: 10.1074/jbc.M111.302042. Epub 2011 Oct 17. J Biol Chem. 2011. PMID: 22006917 Free PMC article.
-
The molecular basis of human keratin disorders.Hum Genet. 2009 May;125(4):355-73. doi: 10.1007/s00439-009-0646-5. Epub 2009 Feb 27. Hum Genet. 2009. PMID: 19247691 Review.
-
The Cytoskeleton-A Complex Interacting Meshwork.Cells. 2019 Apr 18;8(4):362. doi: 10.3390/cells8040362. Cells. 2019. PMID: 31003495 Free PMC article. Review.
-
Charcot-Marie-Tooth type 2B disease-causing RAB7A mutant proteins show altered interaction with the neuronal intermediate filament peripherin.Acta Neuropathol. 2013 Feb;125(2):257-72. doi: 10.1007/s00401-012-1063-8. Epub 2012 Nov 23. Acta Neuropathol. 2013. PMID: 23179371 Free PMC article.
-
A mitotic GlcNAcylation/phosphorylation signaling complex alters the posttranslational state of the cytoskeletal protein vimentin.Mol Biol Cell. 2008 Oct;19(10):4130-40. doi: 10.1091/mbc.e07-11-1146. Epub 2008 Jul 23. Mol Biol Cell. 2008. PMID: 18653473 Free PMC article.
References
-
- Herrmann H, Hesse M, Reichenzeller M, Aebi U, Magin TM. Functional complexity of intermediate filament cytoskeletons: from structure to assembly to gene ablation. Int Rev Cytol 2003; 223: 83–175. - PubMed
-
- Coulombe PA, Wong P. Cytoplasmic intermediate filaments revealed as dynamic and multipurpose scaffolds. Nat Cell Biol 2004; 6: 699–706. - PubMed
-
- Fuchs E, Cleveland DW. A structural scaffolding of intermediate filaments in health and disease. Science 1998; 279: 514–19. - PubMed
-
- Omary MB, Coulombe PA, McLean WHI. Intermediate filament proteins and their associated diseases. N Engl J Med 2004; 351: 2087–100. - PubMed
-
- Gruenbaum Y, Margalit A, Goldman RD, Shumaker DK, Wilson KL. The nuclear lamina comes of age. Nat Rev Mol Cell Biol 2005; 6: 21–31. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

