Functional effectiveness of the blood-brain barrier to small water-soluble molecules in developing and adult opossum (Monodelphis domestica)
- PMID: 16528724
- PMCID: PMC2634607
- DOI: 10.1002/cne.20885
Functional effectiveness of the blood-brain barrier to small water-soluble molecules in developing and adult opossum (Monodelphis domestica)
Abstract
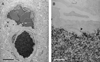
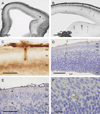
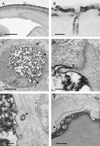
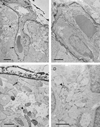
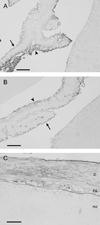
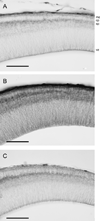
We have evaluated a small water-soluble molecule, biotin ethylenediamine (BED, 286 Da), as a permeability tracer across the blood-brain barrier. This molecule was found to have suitable characteristics in that it is stable in plasma, has low plasma protein binding, and appears to behave in a similar manner across brain barriers as established by permeability markers such as sucrose. BED, together with a 3000-Da biotin-dextran (BDA3000), was used to investigate the effectiveness of tight junctions in cortical vessels during development and adulthood of a marsupial opossum (Monodelphis domestica). Marsupial species are born at an early stage of brain development when cortical vessels are just beginning to appear. The tracers were administered systemically to opossums at various ages and localized in brains with light and electron microscopy. In adults, the tight junctions restricted the movement of both tracers. In neonates, as soon as vessels grow into the neocortex, their tight junctions are functionally restrictive, a finding supported by the presence of claudin-5 in endothelial cells. However, both tracers are also found within brain extracellular space soon after intraperitoneal administration. The main route of entry for the tracers into immature neocortex appears to be via the cerebrospinal fluid over the outer (subarachnoid) and inner (ventricular) surfaces of the brain. These experiments demonstrate that the previously described higher permeability of barriers to small molecules in the developing brain does not seem to be due to leakiness of cerebral endothelial tight junctions, but to a route of entry probably via the choroid plexuses and cerebrospinal fluid.
Figures


Similar articles
-
Permeability and route of entry for lipid-insoluble molecules across brain barriers in developing Monodelphis domestica.J Physiol. 2001 Nov 1;536(Pt 3):841-53. doi: 10.1111/j.1469-7793.2001.00841.x. J Physiol. 2001. PMID: 11691876 Free PMC article.
-
Cellular transfer of macromolecules across the developing choroid plexus of Monodelphis domestica.Eur J Neurosci. 2009 Jan;29(2):253-66. doi: 10.1111/j.1460-9568.2008.06571.x. Eur J Neurosci. 2009. PMID: 19200232
-
Structural characteristics and barrier properties of the choroid plexuses in developing brain of the opossum (Monodelphis Domestica).J Comp Neurol. 2003 Jun 9;460(4):451-64. doi: 10.1002/cne.10661. J Comp Neurol. 2003. PMID: 12717706
-
Molecular mechanisms of brain tumor edema.Neuroscience. 2004;129(4):1011-20. doi: 10.1016/j.neuroscience.2004.05.044. Neuroscience. 2004. PMID: 15561416 Review.
-
Barrier mechanisms in the brain, II. Immature brain.Clin Exp Pharmacol Physiol. 1999 Feb;26(2):85-91. doi: 10.1046/j.1440-1681.1999.02987.x. Clin Exp Pharmacol Physiol. 1999. PMID: 10065326 Review.
Cited by
-
The rights and wrongs of blood-brain barrier permeability studies: a walk through 100 years of history.Front Neurosci. 2014 Dec 16;8:404. doi: 10.3389/fnins.2014.00404. eCollection 2014. Front Neurosci. 2014. PMID: 25565938 Free PMC article. Review.
-
Optimization of volumetric computed tomography for skeletal analysis of model genetic organisms.Anat Rec (Hoboken). 2008 May;291(5):475-87. doi: 10.1002/ar.20670. Anat Rec (Hoboken). 2008. PMID: 18286615 Free PMC article.
-
ABC Efflux Transporters and Solute Carriers in the Early Developing Brain of a Marsupial Monodelphis domestica (South American Gray Short-Tailed Opossum).J Comp Neurol. 2024 Jul;532(7):e25655. doi: 10.1002/cne.25655. J Comp Neurol. 2024. PMID: 38980080
-
Ontogeny of tight junction protein expression in the ovine cerebral cortex during development.Neuroscience. 2015 Dec 3;310:422-9. doi: 10.1016/j.neuroscience.2015.09.062. Epub 2015 Sep 28. Neuroscience. 2015. PMID: 26424381 Free PMC article.
-
Blood-brain barrier disruption: a culprit of cognitive decline?Fluids Barriers CNS. 2024 Aug 7;21(1):63. doi: 10.1186/s12987-024-00563-3. Fluids Barriers CNS. 2024. PMID: 39113115 Free PMC article. Review.
References
-
- Bass NH, Lundborg P. Postnatal development of bulk flow in the cerebrospinal fluid system of the albino rat: clearance of carboxyl-(14C)inulin after intrathecal infusion. Brain Res. 1973;52:323–332. - PubMed
-
- Bauer HC, Bauer H, Lametschwandtner A, Amberger A, Ruiz P, Steiner M. Neovascularization and the appearance of morphological characteristics of the blood-brain barrier in the embryonic mouse central nervous system. Brain Res Dev Brain Res. 1993;75:269–278. - PubMed
-
- Bauer H, Sonnleitner U, Lametschwandtner A, Steiner M, Adam H, Bauer HC. Ontogenic expression of the erythroid-type glucose transporter (Glut 1) in the telencephalon of the mouse: correlation to the tightening of the blood-brain barrier. Brain Res Dev Brain Res. 86:317–325. - PubMed
-
- Claude P. Morphological factors influencing transepithelial permeability: a model for the resistance of the zonula occludens. J Membr Biol. 1978;3:219–232. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

