Pulmonary cytomegalovirus reactivation causes pathology in immunocompetent mice
- PMID: 16521279
- PMCID: PMC1894751
- DOI: 10.1097/01.ccm.0000201876.11059.05
Pulmonary cytomegalovirus reactivation causes pathology in immunocompetent mice
Abstract
Objective: Cytomegalovirus (CMV) is a ubiquitous herpes virus that persists in the host in a latent state following primary infection. We have recently observed that CMV reactivates in lungs of critically ill surgical patients and that this reactivation can be triggered by bacterial sepsis. Although CMV is a known pathogen in immunosuppressed transplant patients, it is unknown whether reactivated CMV is a pathogen in immunocompetent hosts. Using an animal model of latency/reactivation, we studied the pathobiology of CMV reactivation in the immunocompetent host.
Design: Laboratory study.
Setting: University laboratory.
Subjects: Cohorts of immunocompetent BALB/c mice with or without latent murine CMV (MCMV+/MCMV-).
Interventions: Mice underwent cecal ligation and puncture. Lung tissue homogenates were evaluated after cecal ligation and puncture for tumor necrosis factor-alpha, interleukin-1beta, neutrophil chemokine KC, and macrophage inflammatory protein-2 messenger RNA by polymerase chain reaction and real-time quantitative reverse transcription-polymerase chain reaction. Because pulmonary tumor necrosis factor-alpha expression is known to cause pulmonary fibrosis, trichrome-stained sections of lung tissues were analyzed using image analysis to quantitate pulmonary fibrosis. In a second experiment, a cohort of MCMV+ mice received ganciclovir (10 mg/kg/day subcutaneously) following cecal ligation and puncture. Tumor necrosis factor-alpha messenger RNA and pulmonary fibrosis were evaluated as described previously.
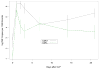
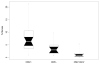
Measurements and main results: All MCMV+ mice had CMV reactivation beginning 2 wks after cecal ligation and puncture. Following reactivation, these mice had abnormal tumor necrosis factor-alpha, interleukin-1beta, neutrophil chemokine KC, and macrophage inflammatory protein-2 messenger RNA expression compared with controls. Image analysis showed that MCMV+ mice had significantly increased pulmonary fibrosis compared with MCMV- mice 3 wks after cecal ligation and puncture. Ganciclovir treatment following cecal ligation and puncture prevented MCMV reactivation. Furthermore, ganciclovir-treated mice did not demonstrate abnormal pulmonary expression of tumor necrosis factor-alpha messenger RNA. Finally, ganciclovir treatment prevented pulmonary fibrosis following MCMV reactivation.
Conclusions: This study shows that CMV reactivation causes abnormal tumor necrosis factor-alpha expression, and that following CMV reactivation, immunocompetent mice have abnormal pulmonary fibrosis. Ganciclovir blocks MCMV reactivation, thus preventing abnormal tumor necrosis factor-alpha expression and pulmonary fibrosis. These data may explain a mechanism by which critically ill surgical patients develop fibroproliferative acute respiratory distress syndrome. These data suggest that human studies using antiviral agents during critical illness are warranted.
Figures






Comment in
-
How cytomegalorvirus reactivation could cause pulmonary pathology in septic hosts.Crit Care Med. 2006 Mar;34(3):929-30. doi: 10.1097/01.CCM.0000202442.33792.04. Crit Care Med. 2006. PMID: 16505692 No abstract available.
Similar articles
-
Lipopolysaccharide, tumor necrosis factor alpha, or interleukin-1beta triggers reactivation of latent cytomegalovirus in immunocompetent mice.J Virol. 2006 Sep;80(18):9151-8. doi: 10.1128/JVI.00216-06. J Virol. 2006. PMID: 16940526 Free PMC article.
-
Intra-abdominal bacterial infection reactivates latent pulmonary cytomegalovirus in immunocompetent mice.J Infect Dis. 2002 May 15;185(10):1395-400. doi: 10.1086/340508. Epub 2002 Apr 30. J Infect Dis. 2002. PMID: 11992273
-
How cytomegalorvirus reactivation could cause pulmonary pathology in septic hosts.Crit Care Med. 2006 Mar;34(3):929-30. doi: 10.1097/01.CCM.0000202442.33792.04. Crit Care Med. 2006. PMID: 16505692 No abstract available.
-
A model for reactivation of CMV from latency.J Clin Virol. 2002 Aug;25 Suppl 2:S123-36. doi: 10.1016/s1386-6532(02)00088-4. J Clin Virol. 2002. PMID: 12361763 Review.
-
[Citomegalovirus reactivation in critical ill intensive care patients].Gac Med Mex. 2011 Mar-Apr;147(2):159-62. Gac Med Mex. 2011. PMID: 21527972 Review. Spanish.
Cited by
-
The pathogenetic role of CMV in intensive care unit patients: the uncertainity remains.J Thorac Dis. 2017 Jul;9(7):1780-1782. doi: 10.21037/jtd.2017.06.79. J Thorac Dis. 2017. PMID: 28839961 Free PMC article. No abstract available.
-
Highly quantitative serological detection of anti-cytomegalovirus (CMV) antibodies.Virol J. 2009 May 1;6:45. doi: 10.1186/1743-422X-6-45. Virol J. 2009. PMID: 19409090 Free PMC article.
-
Cytomegalovirus: A Troll in the ICU? Overview of the Literature and Perspectives for the Future.Front Med (Lausanne). 2020 May 15;7:188. doi: 10.3389/fmed.2020.00188. eCollection 2020. Front Med (Lausanne). 2020. PMID: 32500076 Free PMC article.
-
Antiviral prophylaxis of cytomegalovirus reactivation in immune competent patients-the jury remains out.J Thorac Dis. 2017 Aug;9(8):2221-2223. doi: 10.21037/jtd.2017.06.130. J Thorac Dis. 2017. PMID: 28932509 Free PMC article. No abstract available.
-
Cytomegalovirus reactivation and associated outcome of critically ill patients with severe sepsis.Crit Care. 2011;15(2):R77. doi: 10.1186/cc10069. Epub 2011 Mar 1. Crit Care. 2011. PMID: 21362193 Free PMC article.
References
-
- Simmons RL, et al. Clinical characteristics of the lethal cytomegalovirus infection following renal transplantation. Surgery. 1977;82(5):537–46. - PubMed
-
- Cook CH, et al. Occult herpes family viruses may increase mortality in critically ill surgical patients. American Journal of Surgery. 1998;176(4):357–60. - PubMed
-
- Cook CH, et al. Occult herpes family viral infections are endemic in critically ill surgical patients. Critical Care Medicine. 2003;31(7):1923–9. - PubMed
-
- Heininger A, et al. Human cytomegalovirus infections in nonimmunosuppressed critically ill patients. Critical Care Medicine. 2001;29(3):541–7. [see comments] - PubMed
-
- Curtsinger LJ, et al. Association of cytomegalovirus infection with increased morbidity is independent of transfusion. The American Journal of Surgery. 1989;158(6):606–611. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

