A regulated nucleocytoplasmic shuttle contributes to Bright's function as a transcriptional activator of immunoglobulin genes
- PMID: 16507996
- PMCID: PMC1430300
- DOI: 10.1128/MCB.26.6.2187-2201.2006
A regulated nucleocytoplasmic shuttle contributes to Bright's function as a transcriptional activator of immunoglobulin genes
Abstract
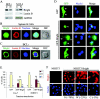
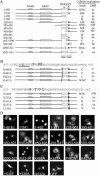
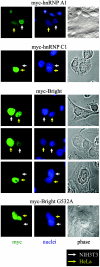
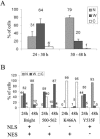
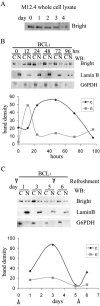
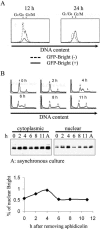
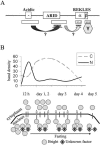
Bright/ARID3a has been implicated in mitogen- and growth factor-induced up-regulation of immunoglobulin heavy-chain (IgH) genes and in E2F1-dependent G1/S cell cycle progression. For IgH transactivation, Bright binds to nuclear matrix association regions upstream of certain variable region promoters and flanking the IgH intronic enhancer. While Bright protein was previously shown to reside within the nuclear matrix, we show here that a significant amount of Bright resides in the cytoplasm of normal and transformed B cells. Leptomycin B, chromosome region maintenance 1 (CRM1) overexpression, and heterokaryon experiments indicate that Bright actively shuttles between the nucleus and the cytoplasm in a CRM1-dependent manner. We mapped the functional nuclear localization signal to the N-terminal region of REKLES, a domain conserved within ARID3 paralogues. Residues within the C terminus of REKLES contain its nuclear export signal, whose regulation is primarily responsible for Bright shuttling. Growth factor depletion and cell synchronization experiments indicated that Bright shuttling during S phase of the cell cycle leads to an increase in its nuclear abundance. Finally, we show that shuttle-incompetent Bright point mutants, even if sequestered within the nucleus, are incapable of transactivating an IgH reporter gene. Therefore, regulation of Bright's cellular localization appears to be required for its function.
Figures


Similar articles
-
REKLES is an ARID3-restricted multifunctional domain.J Biol Chem. 2007 May 25;282(21):15768-77. doi: 10.1074/jbc.M700397200. Epub 2007 Mar 29. J Biol Chem. 2007. PMID: 17400556
-
Keap1 regulates the oxidation-sensitive shuttling of Nrf2 into and out of the nucleus via a Crm1-dependent nuclear export mechanism.Mol Cell Biol. 2005 Jun;25(11):4501-13. doi: 10.1128/MCB.25.11.4501-4513.2005. Mol Cell Biol. 2005. PMID: 15899855 Free PMC article.
-
Nucleocytoplasmic shuttling of Smad1 conferred by its nuclear localization and nuclear export signals.J Biol Chem. 2001 Oct 19;276(42):39404-10. doi: 10.1074/jbc.M103117200. Epub 2001 Aug 16. J Biol Chem. 2001. PMID: 11509558
-
Regulating inducible transcription through controlled localization.Sci STKE. 2005 May 17;2005(284):re6. doi: 10.1126/stke.2842005re6. Sci STKE. 2005. PMID: 15900032 Review.
-
Nuclear-cytoplasmic Shuttling in Chronic Myeloid Leukemia: Implications in Leukemia Maintenance and Therapy.Cells. 2019 Oct 14;8(10):1248. doi: 10.3390/cells8101248. Cells. 2019. PMID: 31614958 Free PMC article. Review.
Cited by
-
Androgen receptor activity is affected by both nuclear matrix localization and the phosphorylation status of the heterogeneous nuclear ribonucleoprotein K in anti-androgen-treated LNCaP cells.PLoS One. 2013 Nov 13;8(11):e79212. doi: 10.1371/journal.pone.0079212. eCollection 2013. PLoS One. 2013. PMID: 24236111 Free PMC article.
-
Arid3a regulates mesoderm differentiation in mouse embryonic stem cells.J Stem Cell Ther Transplant. 2017;1(1):52-62. doi: 10.29328/journal.jsctt.1001005. Epub 2017 Sep 7. J Stem Cell Ther Transplant. 2017. PMID: 31080945 Free PMC article.
-
The ARID family transcription factor bright is required for both hematopoietic stem cell and B lineage development.Mol Cell Biol. 2011 Mar;31(5):1041-53. doi: 10.1128/MCB.01448-10. Epub 2011 Jan 3. Mol Cell Biol. 2011. PMID: 21199920 Free PMC article.
-
Characterization of a new ARID family transcription factor (Brightlike/ARID3C) that co-activates Bright/ARID3A-mediated immunoglobulin gene transcription.Mol Immunol. 2011 Oct;49(1-2):260-72. doi: 10.1016/j.molimm.2011.08.025. Epub 2011 Sep 28. Mol Immunol. 2011. PMID: 21955986 Free PMC article.
-
Arid3c identifies an uncharacterized subpopulation of V2 interneurons during embryonic spinal cord development.Front Cell Neurosci. 2024 Oct 16;18:1466056. doi: 10.3389/fncel.2024.1466056. eCollection 2024. Front Cell Neurosci. 2024. PMID: 39479525 Free PMC article.
References
-
- Alt, J. R., A. B. Gladden, and J. A. Diehl. 2002. p21(Cip1) promotes cyclin D1 nuclear accumulation via direct inhibition of nuclear export. J. Biol. Chem. 277:8517-8523. - PubMed
-
- Avitahl, N., and K. A. Calame. 1996. 125 bp region of the Ig VH1 promoter is sufficient to confer lymphocyte-specific expression in transgenic mice. Int. Immunol. 8:1359-1366. - PubMed
-
- Blanc, V., S. Kennedy, and N. O. Davidson. 2003. A novel nuclear localization signal in the auxiliary domain of apobec-1 complementation factor regulates nucleocytoplasmic import and shuttling. J. Biol. Chem. 278:41198-41204. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
