Intensified and protective CD4+ T cell immunity in mice with anti-dendritic cell HIV gag fusion antibody vaccine
- PMID: 16505141
- PMCID: PMC2118242
- DOI: 10.1084/jem.20052005
Intensified and protective CD4+ T cell immunity in mice with anti-dendritic cell HIV gag fusion antibody vaccine
Abstract
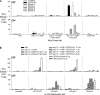
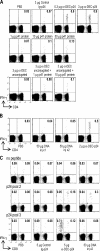
Current human immunodeficiency virus (HIV) vaccine approaches emphasize prime boost strategies comprising multiple doses of DNA vaccine and recombinant viral vectors. We are developing a protein-based approach that directly harnesses principles for generating T cell immunity. Vaccine is delivered to maturing dendritic cells in lymphoid tissue by engineering protein antigen into an antibody to DEC-205, a receptor for antigen presentation. Here we characterize the CD4+ T cell immune response to HIV gag and compare efficacy with other vaccine strategies in a single dose. DEC-205-targeted HIV gag p24 or p41 induces stronger CD4+ T cell immunity relative to high doses of gag protein, HIV gag plasmid DNA, or recombinant adenovirus-gag. High frequencies of interferon (IFN)-gamma- and interleukin 2-producing CD4+ T cells are elicited, including double cytokine-producing cells. In addition, the response is broad because the primed mice respond to an array of peptides in different major histocompatibility complex haplotypes. Long-lived T cell memory is observed. After subcutaneous vaccination, CD4+ and IFN-gamma-dependent protection develops to a challenge with recombinant vaccinia-gag virus at a mucosal surface, the airway. We suggest that a DEC-targeted vaccine, in part because of an unusually strong and protective CD4+ T cell response, will improve vaccine efficacy as a stand-alone approach or with other modalities.
Figures


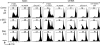

Similar articles
-
A dendritic cell targeted vaccine induces long-term HIV-specific immunity within the gastrointestinal tract.Mucosal Immunol. 2016 Sep;9(5):1340-52. doi: 10.1038/mi.2015.133. Epub 2016 Jan 6. Mucosal Immunol. 2016. PMID: 26732678 Free PMC article.
-
Improved cellular and humoral immune responses in vivo following targeting of HIV Gag to dendritic cells within human anti-human DEC205 monoclonal antibody.Blood. 2010 Nov 11;116(19):3828-38. doi: 10.1182/blood-2010-06-288068. Epub 2010 Jul 28. Blood. 2010. PMID: 20668230 Free PMC article.
-
Enhancement of the priming efficacy of DNA vaccines encoding dendritic cell-targeted antigens by synergistic toll-like receptor ligands.BMC Immunol. 2009 Aug 3;10:43. doi: 10.1186/1471-2172-10-43. BMC Immunol. 2009. PMID: 19650904 Free PMC article.
-
Dendritic cell-targeted protein vaccines: a novel approach to induce T-cell immunity.J Intern Med. 2012 Feb;271(2):183-92. doi: 10.1111/j.1365-2796.2011.02496.x. Epub 2012 Jan 4. J Intern Med. 2012. PMID: 22126373 Free PMC article. Review.
-
Use of adenovirus in vaccines for HIV.Handb Exp Pharmacol. 2009;(188):275-93. doi: 10.1007/978-3-540-71029-5_13. Handb Exp Pharmacol. 2009. PMID: 19031031 Review.
Cited by
-
Targeting CLEC9A delivers antigen to human CD141+ DC for CD4+ and CD8+T cell recognition.JCI Insight. 2016 May 19;1(7):e87102. doi: 10.1172/jci.insight.87102. JCI Insight. 2016. PMID: 27699265 Free PMC article.
-
An IFN-gamma-IL-18 signaling loop accelerates memory CD8+ T cell proliferation.PLoS One. 2008 Jun 11;3(6):e2404. doi: 10.1371/journal.pone.0002404. PLoS One. 2008. PMID: 18545704 Free PMC article.
-
Regulation of Dendritic Cell Function in Inflammation.J Immunol Res. 2015;2015:743169. doi: 10.1155/2015/743169. Epub 2015 Jul 2. J Immunol Res. 2015. PMID: 26229971 Free PMC article. Review.
-
Potentiating functional antigen-specific CD8⁺ T cell immunity by a novel PD1 isoform-based fusion DNA vaccine.Mol Ther. 2013 Jul;21(7):1445-55. doi: 10.1038/mt.2013.63. Epub 2013 Apr 16. Mol Ther. 2013. PMID: 23587922 Free PMC article.
-
DEC-205/CD205+ dendritic cells are abundant in the white pulp of the human spleen, including the border region between the red and white pulp.Immunology. 2008 Mar;123(3):438-46. doi: 10.1111/j.1365-2567.2007.02710.x. Epub 2007 Oct 16. Immunology. 2008. PMID: 17944899 Free PMC article.
References
-
- Seder, R.A., and J.R. Mascola. 2003. Basic immunology of vaccine development. In The Vaccine Book. B.R. Bloom and P.-H. Lambert, editors. Academic Press, Boston. 51–72 pp.
-
- Pantaleo, G., and R.A. Koup. 2004. Correlates of immune protection in HIV-1 infection: what we know, what we don't know, what we should know. Nat. Med. 10:806–810. - PubMed
-
- Saul, A., V. Nussenzweig, and R.S. Nussenzweig. 2004. Rationale for malaria vaccine development. In Novel Vaccination Strategies. S.H.E. Kaufmann, editor. Wiley-Vch Verlag GmbH & Co. 479–503.
-
- Frazer, I.H. 2004. Prevention of cervical cancer through papillomavirus vaccination. Nat. Rev. Immunol. 4:46–54. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

